Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0R9OH
|
||||
| Former ID |
DAP001239
|
||||
| Drug Name |
Sulfamethoxazole
|
||||
| Synonyms |
Bactrimel; Gamazole; Gantanol; Metoxal; Radonil; SMX; Septran; Simsinomin; Sinomin; Solfametossazolo; Sulfamethalazole; Sulfamethoxazol; Sulfamethoxazolum; Sulfamethoxizole; Sulfamethylisoxazole; Sulfametoxazol; Sulfisomezole; Sulphamethalazole; Sulphamethoxazol; Sulphamethoxazole; Sulphamethylisoxazole; Sulphisomezole; Trib; Urobak; Solfametossazolo [DCIT]; Sulfamethoxazole sodium; MS 53; Sulphamethoxazole BP 98; ALBB-002089; Apo-Sulfamethoxazole; Azo-gantanol; Bactrim (TN); Gantanol-DS; Ro 4-2130; Septra (TN); Septrin (TN); Sulfamethoxazolum [INN-Latin]; Sulfametoxazol [INN-Spanish]; Sulpha-methoxizole; Ro 6-2580/11; Ro-4-2130; Sulfamethoxazole [USAN:INN:JAN]; TMP/SMX (MIXTURE)); Sulfamethoxazole (JP15/USP/INN); N1-(5-Methyl-3-isoxazolyl)sulfanilamide; N1-(5-Methylisoxazol-3-yl)sulfanilamide; SULFAMETHOXAZOLE (8064-90-2 (TRIMETHOPRIM/SULFAMETHOXAZOLE); N'-(5-Methyl-3-isoxazole)sulfanilamide; N'-(5-Methyl-3-isoxazolyl)sulfanilamide; N'-(5-Methylisoxazol-3-yl)sulphanilamide; N(sup 1)-(5-Methyl-3-isoxazolyl)sulfanilamide; N(sup 1)-(5-Methyl-3-isoxazolyl)sulphanilamide; N(sup1)-(5-Methyl-3-isoxazolyl)sulfanilamide; Sulfanilamide, N1-(5-methyl-3-isoxazolyl)-(8CI); 3-(p-Aminophenylsulfonamido)-5-methylisoxazole; 3-(para-Aminophenylsulphonamido)-5-methylisoxazole; 3-Sulfanilamido-5-methylisoxazole; 3-Sulphanilamido-5-methylisoxazole; 4-Amino-N-(5-methyl-3-isoxazolyl)benzenesulfonamide; 4-Amino-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide; 4-Amino-N-[5-methyl-3-isoxazolyl]benzenesulfonamide; 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide; 4-amino-N-(5-methylisoxazol-3-yl)benzenesulfonamide; 5-Methyl-3-sulfanilamidoisoxazole; 5-Methyl-3-sulfanylamidoisoxazole; 5-Methyl-3-sulphanil-amidoisoxazole
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiinfective Agents
|
||||
| Structure |
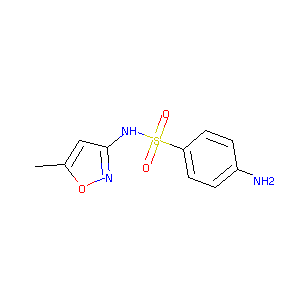
|
Download2D MOL |
|||
| Formula |
C10H11N3O3S
|
||||
| Canonical SMILES |
CC1=CC(=NO1)NS(=O)(=O)C2=CC=C(C=C2)N
|
||||
| InChI |
1S/C10H11N3O3S/c1-7-6-10(12-16-7)13-17(14,15)9-4-2-8(11)3-5-9/h2-6H,11H2,1H3,(H,12,13)
|
||||
| InChIKey |
JLKIGFTWXXRPMT-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 723-46-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9523, 430150, 602530, 855654, 3160889, 4484561, 7847513, 7980702, 8149537, 8153272, 10321458, 10533933, 10589657, 11112438, 11335824, 11361063, 11363888, 11366450, 11369012, 11371628, 11373888, 11377174, 11385461, 11462035, 11466205, 11467325, 11484632, 11485730, 11488604, 11490400, 11492160, 11494808, 14718462, 14798729, 17388720, 24870521, 24899752, 25623303, 26611929, 26679802, 26746946, 26746947, 29224383, 46508111, 47188230, 47216775, 47291127, 47365185, 47440251, 47662281
|
||||
| ChEBI ID |
ChEBI:9332
|
||||
| SuperDrug ATC ID |
J01EC01
|
||||
| SuperDrug CAS ID |
cas=000723466
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Dihydropteroate synthetase | Target Info | Inhibitor | [537865] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

