Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0R9US
|
||||
| Former ID |
DIB012665
|
||||
| Drug Name |
GSK2140944
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Phase 2 | [1] | ||
| Company |
Glaxosmithkline
|
||||
| Structure |
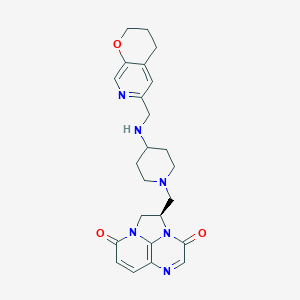
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | DNA topoisomerase II | Target Info | Inhibitor | [2] | |
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02045797) Dose-Ranging Study of GSK2140944 in the Treatment of Subjects With Suspected or Confirmed Gram-Positive Acute Bacterial Skin and Skin Structure Infections. U.S. National Institutes of Health. | ||||
| REF 2 | Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent. J Clin Microbiol. 2014 Jul;52(7):2629-32. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

