Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0S5WG
|
||||
| Former ID |
DAP001542
|
||||
| Drug Name |
Flucytosine
|
||||
| Synonyms |
Alcobon; Ancobon; Ancotil; Ancotyl; Flucitosina; Flucystine; Flucytosin; Flucytosinum; Flucytosone; Fluocytosine; Fluorcytosine; Fluorocytosine; Flucitosina [DCIT]; F0321; LT00771985; Ancobon (TN); Flucytosinum [INN-Latin]; GL663142 & 5FC; Ro 2-9915; Ro 29915 E/265601; Ro-2-9915; Flucytosine (JP15/USP/INN); Flucytosine [USAN:INN:BAN:JAN]; Cytosine, 5-fluoro-(6CI,7CI,8CI); GL663142 & 4-Amino-5-fluoropyrimidin-2(1H)-one; 2(1H)-Pyrimidinone, 4-amino-5-fluoro-); 2-Hydroxy-4-amino-5-fluoropyrimidine; 4-Amino-5-fluoro-2(1H)-pyrimidinone; 4-Amino-5-fluoro-2-hydroxypyrimidine; 4-Amino-5-fluoro-2-hyroxypyrimidine; 4-Amino-5-fluoropyrimidin-2(1H)-one; 5-FC; 5-Flucytosine; 5-Fluorocystosine; 5-Fluorocytosin; 5-Fluorocytosine; 5-Fluorocytosine-6-3H; 5-Flurocytosine; 5-fluoro cytosine; 5987P; 6-Amino-2-oxo-5-fluoropyrimidine; 6-amino-5-fluoro-1H-pyrimidin-2-one; 9074P
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antifungal Agents
|
||||
| Structure |
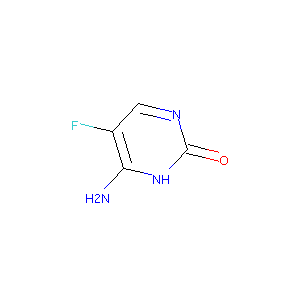
|
Download2D MOL |
|||
| Formula |
C4H4FN3O
|
||||
| Canonical SMILES |
C1=NC(=O)NC(=C1F)N
|
||||
| InChI |
1S/C4H4FN3O/c5-2-1-7-4(9)8-3(2)6/h1H,(H3,6,7,8,9)
|
||||
| InChIKey |
XRECTZIEBJDKEO-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 2022-85-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
405592, 602763, 841044, 1434447, 3140368, 3218074, 5270497, 5329900, 5431724, 6041883, 7847389, 7979233, 8139861, 11112505, 11404488, 11466962, 11468082, 11486773, 11564865, 14747633, 15194483, 24712291, 24870520, 24894919, 24894928, 26719629, 29222501, 39910157, 46386766, 46504735, 47662407, 47736597, 48110551, 48110552, 48416018, 49699216, 49831643, 49960195, 51074762, 53653662, 56424024, 57321755, 57930982, 81041221, 83591697, 85086121, 87570095, 87633378, 88817477, 92125834
|
||||
| ChEBI ID |
ChEBI:5100
|
||||
| SuperDrug ATC ID |
D01AE21; J02AX01
|
||||
| SuperDrug CAS ID |
cas=002022857
|
||||
| Target and Pathway | |||||
| Target(s) | Thymidylate synthase | Target Info | Inhibitor | [536275] | |
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

