Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0S6ME
|
||||
| Former ID |
DNC012995
|
||||
| Drug Name |
6-benzyl-3-propoxycarbonyl-4-quinolone
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
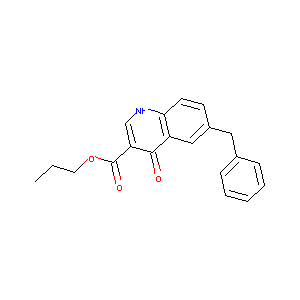
|
Download2D MOL |
|||
| Formula |
C20H19NO3
|
||||
| Canonical SMILES |
CCCOC(=O)C1=CNC2=C(C1=O)C=C(C=C2)CC3=CC=CC=C3
|
||||
| InChI |
1S/C20H19NO3/c1-2-10-24-20(23)17-13-21-18-9-8-15(12-16(18)19(17)22)11-14-6-4-3-5-7-14/h3-9,12-13H,2,10-11H2,1H3,(H,21,22)
|
||||
| InChIKey |
JIOMLDXNFGNQFW-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor subunit alpha-1 | Target Info | Inhibitor | [1] | |
| Gamma-aminobutyric acid receptor subunit beta-2 | Target Info | Inhibitor | [1] | ||
| Gamma-aminobutyric acid receptor | Target Info | Inhibitor | [1] | ||
| Gamma-aminobutyric acid receptor subunit gamma-2 | Target Info | Inhibitor | [1] | ||
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | |||||
| GABAergic synapse | |||||
| Morphine addiction | |||||
| Nicotine addictionhsa04080:Neuroactive ligand-receptor interaction | |||||
| Serotonergic synapse | |||||
| Nicotine addiction | |||||
| Reactome | Ligand-gated ion channel transport | ||||
| GABA A receptor activationR-HSA-975298:Ligand-gated ion channel transport | |||||
| GABA A receptor activation | |||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transportWP2754:Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transport | |||||
| References | |||||
| REF 1 | J Med Chem. 2006 Apr 20;49(8):2526-33.4-quinolone derivatives: high-affinity ligands at the benzodiazepine site of brain GABA A receptors. synthesis, pharmacology, and pharmacophore modeling. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

