Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0SR4G
|
||||
| Former ID |
DIB005924
|
||||
| Drug Name |
ONT-093
|
||||
| Synonyms |
MDR inhibitors, Ontogen; OC-144-093
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Discontinued in Phase 1 | [546965] | ||
| Company |
Ontogen Corp
|
||||
| Structure |
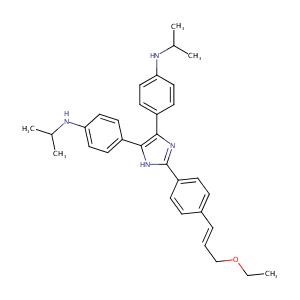
|
Download2D MOL |
|||
| Formula |
C32H38N4O
|
||||
| Canonical SMILES |
n1c(c([nH]c1c1ccc(/C=C/COCC)cc1)c1ccc(NC(C)C)cc1)c1ccc(<br />NC(C)C)cc1
|
||||
| CAS Number |
CAS 216227-54-2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Multidrug resistance protein 1 | Target Info | Inhibitor | [527635] | |
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TCR Signaling Pathway | |||||
| Pathway Interaction Database | HIF-1-alpha transcription factor network | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

