Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T6TW
|
||||
| Former ID |
DCL000825
|
||||
| Drug Name |
GSK642444
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Chronic obstructive pulmonary disease [ICD9: 490-492, 494-496; ICD10:J40-J44, J47] | Phase 3 | [537130] | ||
| Company |
Theravance; GlaxoSmithKline
|
||||
| Structure |
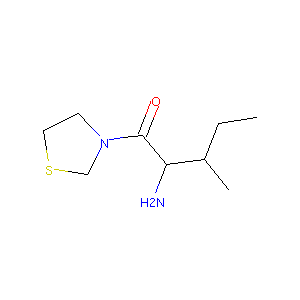
|
Download2D MOL |
|||
| Formula |
C9H19N2OS+
|
||||
| Canonical SMILES |
CCC(C)C(C(=O)N1CCSC1)[NH3+]
|
||||
| InChI |
1S/C9H18N2OS/c1-3-7(2)8(10)9(12)11-4-5-13-6-11/h7-8H,3-6,10H2,1-2H3/p+1/t7-,8-/m0/s1
|
||||
| InChIKey |
WCRLBFHWFPELKW-YUMQZZPRSA-O
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Beta-2 adrenergic receptor | Target Info | Agonist | [532150], [532651], [537130] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | ||||
| Arf6 signaling events | |||||
| References | |||||
| Ref 532150 | Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. PulmPharmacol Ther. 2013 Apr;26(2):256-64. | ||||
| Ref 532651 | 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85-9. | ||||
| Ref 537130 | Emerging drugs in chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2009 Mar;14(1):181-94. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

