Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V0CJ
|
||||
| Former ID |
DNCL002647
|
||||
| Drug Name |
CO-101
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Metastatic pancreatic [ICD10:C25.9] | Phase 2 | [1] | ||
| Company |
Clovis Oncology
|
||||
| Structure |
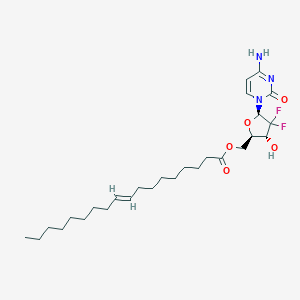
|
Download2D MOL |
|||
| Formula |
C11H13NO3
|
||||
| Canonical SMILES |
CN(C)C(=O)C1=CC=CC=C1C(=O)OC
|
||||
| InChI |
1S/C11H13NO3/c1-12(2)10(13)8-6-4-5-7-9(8)11(14)15-3/h4-7H,1-3H3
|
||||
| InChIKey |
HJPZFXYWMRWNHV-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Human DNA | Target Info | Inhibitor | [2] | |
| Ribonucleoside-diphosphatereductase subunit M2 | Target Info | Inhibitor | [2] | ||
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Guanosine nucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of purine nucleotide salvage | |||||
| Purine nucleotides de novo biosynthesis | |||||
| Adenosine deoxyribonucleotides de novo biosynthesis | |||||
| Guanosine deoxyribonucleotides de novo biosynthesis | |||||
| KEGG Pathway | Purine metabolism | ||||
| Pyrimidine metabolism | |||||
| Glutathione metabolism | |||||
| Metabolic pathways | |||||
| p53 signaling pathway | |||||
| NetPath Pathway | EGFR1 Signaling Pathway | ||||
| PANTHER Pathway | p53 pathway | ||||
| De novo purine biosynthesis | |||||
| De novo pyrimidine deoxyribonucleotide biosynthesis | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Purine Metabolism | ||||
| Pyrimidine Metabolism | |||||
| Reactome | E2F mediated regulation of DNA replication | ||||
| G1/S-Specific Transcription | |||||
| WikiPathways | Nucleotide Metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01233375) Study to Evaluate Efficacy of CO-1.01 as Second Line Therapy for Gemcitabine-Refractory Stage IV Pancreatic Adenocarcinoma. U.S. National Institutes of Health. | ||||
| REF 2 | Cellular pharmacology of gemcitabine. Ann Oncol. 2006 May;17 Suppl 5:v7-12. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

