Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V6OA
|
||||
| Former ID |
DNCL002125
|
||||
| Drug Name |
Lurbinectedin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Leukemia [ICD9: 208.9; ICD10:C90-C95] | Phase 3 | [1] | ||
| Company |
PharmaMar
|
||||
| Structure |
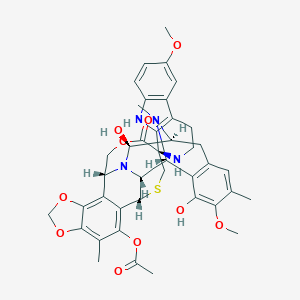
|
Download2D MOL |
|||
| Formula |
C41H44N4O10S
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Human DNA | Target Info | Inhibitor | [2] | |
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02421588) Clinical Trial of Lurbinectedin (PM01183) in Platinum Resistant Ovarian Cancer Patients. U.S. National Institutes of Health. | ||||
| REF 2 | Lurbinectedin (PM01183), a new DNA minor groove binder, inhibits growth of orthotopic primary graft of cisplatin-resistant epithelial ovarian cancer. Clin Cancer Res. 2012 Oct 1;18(19):5399-411. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

