Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0VC5L
|
||||
| Former ID |
DCL000666
|
||||
| Drug Name |
Zunrisa/Rezonic
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Chemotherapy-induced nausea [ICD9: 787, 787.0; ICD10:R11] | Discontinued in Phase 3 | [550098] | ||
| Company |
GSK
|
||||
| Structure |
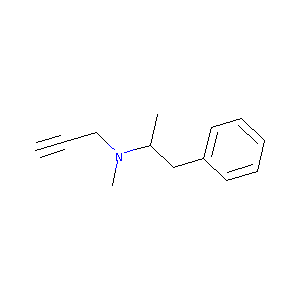
|
Download2D MOL |
|||
| Formula |
C13H17N
|
||||
| Canonical SMILES |
CC(CC1=CC=CC=C1)N(C)CC#C
|
||||
| InChI |
1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1
|
||||
| InChIKey |
MEZLKOACVSPNER-GFCCVEGCSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9454, 7980587, 8169367, 11113910, 11466580, 11467700, 11486280, 14772861, 14797266, 17397820, 26752066, 29293584, 46509459, 47662432, 47810884, 48259382, 48259383, 48334634, 48416534, 49698549, 49880108, 50104720, 50104721, 50139277, 53789537, 57310317, 61127936, 80766630, 85787938, 85788262, 89449661, 92308745, 92310005, 94569169, 99300917, 103252336, 104298196, 104829538, 117391911, 117884350, 128586172, 134337870, 134339187, 134989154, 137001242, 142170639, 160964372, 175267457, 176484098, 176484773
|
||||
| SuperDrug ATC ID |
N04BD01
|
||||
| SuperDrug CAS ID |
cas=014611519
|
||||
| Target and Pathway | |||||
| Target(s) | Substance-P receptor | Target Info | Antagonist | [550963] | |
| PANTHER Pathway | CCKR signaling map ST | ||||
| Reactome | G alpha (q) signalling events | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

