Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0VM2L
|
||||
| Former ID |
DAP001023
|
||||
| Drug Name |
Zoledronate
|
||||
| Synonyms |
Aclasta; Reclast; ZOL; Zometa; Novartis brand of zoledronic acid; Zoledronic acid; Zometa Concentrate; Bisphosphonate 3; CGP 42446; CGP 42446A; Aclasta (TN); CGP 42'446; CGP-42446; KS-1132; Reclast (TN); Zoledronic acid (INN); Zoledronic acid [USAN:INN]; Zomera (TN); Zometa (Novartis); Zometa (TN); CGP-42'446; Zometa, Zomera, Aclasta and Reclast, Zoledronic Acid; [1-hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic acid); (1-Hydroxy-2-imidazol-1-ylethylidene)diphosphonic acid; (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bisphosphonic acid; (1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic acid; (1-hydroxy-2-imidazol-1-yl-phosphonoethyl)phosphonic acid monohydrate; 2-(imidazol-1-yl)-1-hydroxyethane-1,1-diphosphonic acid; 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Hypercalcemia [ICD9: 275.42; ICD10:E83.5] | Approved | [536957] | ||
| Therapeutic Class |
Bone Density Conservation Agents
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
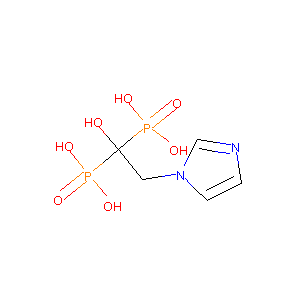
|
Download2D MOL |
|||
| Formula |
C5H10N2O7P2
|
||||
| Canonical SMILES |
C1=CN(C=N1)CC(O)(P(=O)(O)O)P(=O)(O)O
|
||||
| InChI |
1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14)
|
||||
| InChIKey |
XRASPMIURGNCCH-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 118072-93-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
536160, 6436371, 7891233, 7980916, 8192311, 10318362, 10318375, 10318390, 10318944, 11528905, 14824151, 25819963, 29215499, 29215500, 43125267, 46507310, 46513035, 50473172, 53788750, 53801236, 56311814, 56436259, 56479320, 57317038, 81092860, 85174457, 87457600, 89736141, 92308965, 92712542, 93166450, 93167152, 96025372, 99343600, 99343604, 99437087, 99455519, 103240897, 104342928, 109692939, 117626547, 118048654, 124757161, 125163965, 125338822, 126592903, 126625470, 126656631, 126667014, 127324075
|
||||
| ChEBI ID |
ChEBI:46557
|
||||
| SuperDrug ATC ID |
M05BA08
|
||||
| SuperDrug CAS ID |
cas=118072938
|
||||
| Target and Pathway | |||||
| Target(s) | Farnesyl pyrophosphate synthase | Target Info | Modulator | [556264] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | Cholesterol biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

