Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0X4RN
|
||||
| Former ID |
DAP000121
|
||||
| Drug Name |
Lidocaine
|
||||
| Synonyms |
Alphacaine; Anestacon; Cappicaine; Cuivasil; Dalcaine; Dentipatch; DermaFlex; Dilocaine; Duncaine; EMBOLEX; Esracaine; Gravocain; Isicaina; Isicaine; Jetocaine; LIDOPEN; LQZ; Lanabiotic; Leostesin; Lidocaina; Lidocainum; Lidocaton; Lidoderm; Lignocaine; Lignocainum; Lingocaine; Maricaine; Octocaine; Remicaine; Rucaina; Solcain; Xilina; Xilocaina; Xllina; Xycaine; Xylestesin; Xylesthesin; Xylocain; Xylocaine; Xylocard; Xylocitin; Xylotox; Zingo; After Burn Double Strength Gel; After Burn Double Strength Spray; After Burn Gel; After Burn Spray; Anestacon Jelly; Cito optadren; Emla Cream; Lidocaine Carbonate; Lidocaine Hydrocarbonate; Lidocaine Monohydrochloride; Norwood Sunburn Spray; Rocephin Kit; Solarcaine aloe extraburn relief cream; Xilocaina [Italian]; Xylocaine Dental Ointment; Xylocaine Endotracheal; Xylocaine Test Dose; Xylocaine Viscous; CDS1_000283; L1026_SIGMA; Xylocaine CO2; Dentipatch (TN); ELA-Max; L-Caine; Lida-Mantle; Lidocaina [INN-Spanish]; Lidocaine (VAN); Lidocainum [INN-Latin]; Lidoject-1; Lidoject-2; Octocaine-100; Octocaine-50; Xylocaine (TN); Xylocaine 5% Spinal; Xylocaine-Mpf; Xylocaine-Mpf with Glucose; Xyloneural (free base); Zilactin-L; Lidocaine [USAN:INN:JAN]; Diethylaminoaceto-2,6-xylidide; Lidocaine (JP15/USP/INN); Alfa-Dietilamino-2,6-dimetilacetanilide; Alfa-Dietilamino-2,6-dimetilacetanilide [Italian]; Alpha-Diethylamino-2,6-dimethylacetanilide; Alpha-Diethylaminoaceto-2,6-xylidide; LIDOCAINE (73-58-6 (MONOHYDROCHLORIDE); Omega-Diethylamino-2,6-dimethylacetanilide; Alpha-(Diethylamino)-2,6-acetoxylidide; N-(2,6-dimethylphenyl)-N(2),N(2)-diethylglycinamide; N-(2,6-dimethylphenyl)-N~2~,N~2~-diethylglycinamide; 2-(Diethylamino)-2',6'-acetoxylidide; 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide; 2-Diethylamino-N-(2,6-dimethyl-phenyl)-acetamide; 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anesthetics
|
||||
| Company |
Endo Pharmaceuticals
|
||||
| Structure |
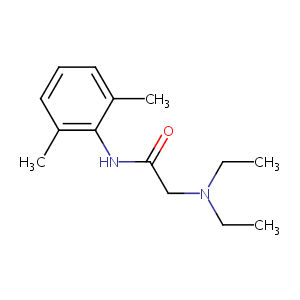
|
Download2D MOL |
|||
| Formula |
C14H22N2O
|
||||
| InChI |
InChI=1S/C14H22N2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4/h7-9H,5-6,10H2,1-4H3,(H,15,17)
|
||||
| InChIKey |
NNJVILVZKWQKPM-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 137-58-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9284, 95410, 855682, 3134587, 5152743, 7847424, 7979776, 8152322, 10507335, 11110520, 11111384, 11111385, 11120259, 11120747, 11121235, 11121424, 11121904, 11335349, 11336498, 11360588, 11362493, 11364012, 11365055, 11366574, 11367617, 11369136, 11370257, 11370258, 11372323, 11373218, 11373738, 11375779, 11377298, 11380842, 11461560, 11466078, 11467198, 11485261, 11485836, 11489159, 11491207, 11491885, 11494932, 11511857, 15196283, 24896272, 24896480, 26719632, 26744219, 26751483
|
||||
| ChEBI ID |
ChEBI:6456
|
||||
| SuperDrug ATC ID |
C01BB01; C05AD01; D04AB01; N01BB02; R02AD02; S01HA07; S02DA01
|
||||
| SuperDrug CAS ID |
cas=000137586
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Voltage-gated sodium channel | Target Info | Blocker | [537243], [537539] | |
| References | |||||
| Ref 522279 | ClinicalTrials.gov (NCT00651313) Efficacy and Safety Study of Lidocaine Vaginal Gel for Recurrent Dysmenorrhea (Painful Periods). U.S. National Institutes of Health. | ||||
| Ref 537084 | An absorbable local anesthetic matrix provides several days of functional sciatic nerve blockade. Anesth Analg. 2009 Mar;108(3):1027-33. | ||||
| Ref 539693 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2623). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

