Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0YA9Z
|
||||
| Former ID |
DAP000432
|
||||
| Drug Name |
Ampicillin
|
||||
| Synonyms |
ABPC; Acillin; Adobacillin; Alpen; Amblosin; Amcill; Amfipen; Aminobenzylpenicillin; Ampen; Ampichel; Ampicil; Ampicilina; Ampicillanyl; Ampicillina; Ampicilline; Ampicillinum; Ampicin; Ampifarm; Ampikel; Ampimed; Ampipenin; Ampiscel; Ampisyn; Ampivax; Ampivet; Amplacilina; Amplin; Amplipenyl; Amplisom; Amplital; Austrapen; Binotal; Bonapicillin; Britacil; Campicillin; Cimex; Copharcilin; Delcillin; Deripen; Divercillin; Doktacillin; Duphacillin; Grampenil; Guicitrina; Guicitrine; Lifeampil; Morepen; Norobrittin; Nuvapen; Omnipen; Orbicilina; Penbristol; Penbritin; Penbrock; Penicline; Penimic; Pensyn; Pentrex; Pentrexl; Pentrexyl; Polycillin; Ponecil; Princillin; Principen; QIDamp; Racenacillin; Rosampline;Roscillin; Semicillin; Servicillin; Sumipanto; Supen; Synpenin; Texcillin; Tokiocillin; Tolomol; Totacillin; Totalciclina; Totapen; Trifacilina; Ukapen; Ultrabion; Ultrabron; Vampen; Viccillin; Wypicil; Amfipen V; Amipenix S; Ampicillin A; Ampicillin Anhydrous; Ampicillin Base; Ampicillin acid; Ampicillin anhydrate; Ampicillina [DCIT]; Anhydrous ampicillin; Olin Kid; Pen A; Pen A Oral; Pen Ampil;Penbritin paediatric; Penbritin syrup; Pfizerpen A; Semicillin R; Viccillin S; AY 6108; BA 7305; BRL 1341; Bayer 5427; HI 63; P 50; Principen 125; Principen 250; Principen 500; SQ 17382; AB-PC; AB-PCSol; AY-6108; Ambidrin (TN); Ampi-Co; Ampi-Tab; Ampi-bol; Ampicilina [INN-Spanish]; Ampicilline [INN-French]; Ampicillinum [INN-Latin]; Ampipenin, nt3; Ampy-Penyl; Anhydrous ampicillin (JP15); BRL-1341; D-Ampicillin; D-Cillin; KS-R1; Novo-ampicillin; OMNIPEN (AMPICILLIN); Omnipen (TN); Omnipen-N; P-50; Penbritin-S; Penicillin, Aminobenzyl; Pfizerpen-A; Polycillin-N; Polyflex (Veterinary); Ro-Ampen; SK-Ampicillin; Totacillin (sodium); Totacillin-N; WY-5103; Ampicillin (USP/INN); AMPICILLIN (SEE ALSO AMPICILLIN TRIHYDRATE 7177-48-2); Ampicillin [USAN:BAN:INN:JAN]; Ampicillin [USAN:INN:BAN:JAN];D-(-)-Ampicillin; D-(-)-alpha-Aminobenzylpenicillin; D-(-)-alpha-Aminopenicillin; D-(-)-6-(alpha-Aminophenylacetamido)penicillanic acid; 6-(D(-)-alpha-Aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [1] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Stadapharm GmbH
|
||||
| Structure |
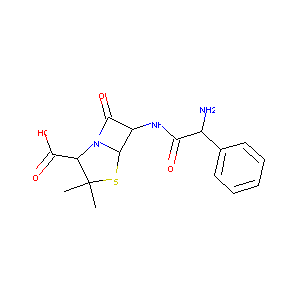
|
Download2D MOL |
|||
| Formula |
C16H19N3O4S
|
||||
| Canonical SMILES |
CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)N)C(=O)O)C
|
||||
| InChI |
1S/C16H19N3O4S/c1-16(2)11(15(22)23)19-13(21)10(14(19)24-16)18-12(20)9(17)8-6-4-3-5-7-8/h3-7,9-11,14H,17H2,1-2H3,(H,18,20)(H,22,23)/t9-,10-,11+,14-/m1/s1
|
||||
| InChIKey |
AVKUERGKIZMTKX-NJBDSQKTSA-N
|
||||
| CAS Number |
CAS 69-53-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
8803, 612819, 855874, 7847272, 7978702, 8153929, 11335641, 11360880, 11362844, 11365406, 11367968, 11371462, 11373737, 11376130, 11461852, 11466142, 11467262, 11484554, 11485803, 11488585, 11490117, 11491964, 11493864, 14802821, 14827659, 24891442, 26706523, 29215015, 29225245, 46392100, 46484751, 46505346, 47291088, 47440199, 47810705, 47885360, 47959687, 48035057, 48035058, 48184948, 48415562, 49698352, 49855717, 50064028, 50104132, 50930328, 53789146, 53801116, 56312591, 56314308
|
||||
| ChEBI ID |
ChEBI:28971
|
||||
| SuperDrug ATC ID |
J01CA01; S01AA19
|
||||
| SuperDrug CAS ID |
cas=000069534
|
||||
| Target and Pathway | |||||
| Target(s) | DNA | Target Info | Binder | [2] | |
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061936. | ||||
| REF 2 | Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob Agents Chemother. 2002 May;46(5):1273-80. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

