| Drug General Information |
| Drug ID |
D0ZV0R
|
| Former ID |
DIB001971
|
| Drug Name |
F-200
|
| Synonyms |
Eos-200-F; Anti-alpha-5/beta-1 integrin Ab Fragment, PDL; Anti-alpha-5/beta-1 integrin Fab, PDL; Anti-alpha-5/beta-1 integrin Fab, Protein Design Labs
|
| Drug Type |
Small molecular drug
|
| Company |
Eos Biotechnology Inc
|
| Structure |
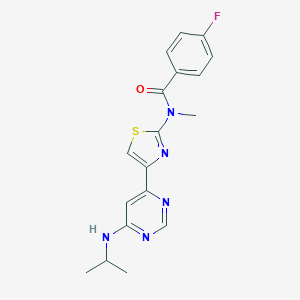
|
Download
2D MOL
3D MOL
|
| Formula |
C22H32Cl2N2
|
| Canonical SMILES |
CC(CC1=CC=CC=C1)N2CCN(CC2)C(C)CC3=CC=CC=C3.Cl.Cl
|
| InChI |
1S/C22H30N2.2ClH/c1-19(17-21-9-5-3-6-10-21)23-13-15-24(16-14-23)20(2)18-22-11-7-4-8-12-22;;/h3-12,19-20H,13-18H2,1-2H3;2*1H
|
| InChIKey |
MQZLPLLJULLBQC-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| PubChem Substance ID |
10226471, 10501672, 44424971, 50011481, 50011482, 50430482, 57335826, 103094391, 103149471, 104412527, 117445509, 135051605, 135178784, 163112731
|
| Target and Pathway |
| References |