| Drug General Information |
| Drug ID |
D05WQF
|
| Former ID |
DNC011169
|
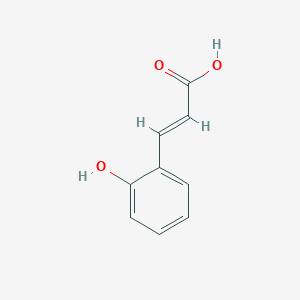
| Drug Name |
2-Hydroxycinnamic acid
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
|
Download
2D MOL
3D MOL
|
| Formula |
C9H8O3
|
| Canonical SMILES |
C1=CC=C(C(=C1)C=CC(=O)O)O
|
| InChI |
1S/C9H8O3/c10-8-4-2-1-3-7(8)5-6-9(11)12/h1-6,10H,(H,11,12)
|
| InChIKey |
PMOWTIHVNWZYFI-UHFFFAOYSA-N
|
| CAS Number |
CAS 614-60-8
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Carbonic anhydrase II |
Target Info |
Inhibitor |
[1]
|
|---|
| Carbonic anhydrase I |
Target Info |
Inhibitor |
[1]
|
|
KEGG Pathway
|
Nitrogen metabolism
|
|
Proximal tubule bicarbonate reclamation
|
|
Collecting duct acid secretion
|
|
Gastric acid secretion
|
|
Pancreatic secretion
|
|
Bile secretionhsa00910:Nitrogen metabolism
|
|
NetPath Pathway
|
IL4 Signaling Pathway
|
|
EGFR1 Signaling Pathway
|
|
Pathway Interaction Database
|
C-MYB transcription factor network
|
|
PathWhiz Pathway
|
Gastric Acid Production
|
|
Reactome
|
Erythrocytes take up carbon dioxide and release oxygen
|
|
Erythrocytes take up oxygen and release carbon dioxide
|
|
Reversible hydration of carbon dioxideR-HSA-1237044:Erythrocytes take up carbon dioxide and release oxygen
|
|
Reversible hydration of carbon dioxide
|
|
WikiPathways
|
Reversible Hydration of Carbon Dioxide
|
|
Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes
|
|
Uptake of Oxygen and Release of Carbon Dioxide by ErythrocytesWP2770:Reversible Hydration of Carbon Dioxide
|
|
Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes
|
| References |
| REF 1 | Bioorg Med Chem Lett. 2010 Sep 1;20(17):5050-3. Epub 2010 Jul 13.Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. |
|---|