Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0X7GL
|
||||
| Former ID |
DAP001422
|
||||
| Drug Name |
Retigabine
|
||||
| Synonyms |
Ezogabine; Potiga; Trobalt; Retigabine [USAN]; D 20443; D 23129; ADD-230001; D-20443; D-23129; GKE-841; KE-0201; Ethyl 2-amino-4-((p-fluorobenzyl)amino)carbanilate; Ethyl N-[2-amino-4-[(4-fluorophenyl)methylamino]phenyl]carbamate; [2-Amino-4-[[(4-fluorophenyl)methyl]amino]phenyl]-carbamate; Ethyl N-(2-amino-4-(4-fluorobenzylamino)phenyl)carbamate hydrochloride; N-(2-Amino-4-(4-fluorobenzylamino)phenyl)carbamic acid ethyl ester; Ethyl (2-amino-4-(((4-fluorophenyl)methyl)amino)phenyl)carbamate; N-(2-Amino-4-(4-fluorobenzylamino)-phenyl) carbamic acid ethyl ester; Carbamic acid, (2-amino-4-(((4-fluorophenyl)methyl)amino)phenyl)-, ethyl ester
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticonvulsants
|
||||
| Company |
GSK
|
||||
| Structure |
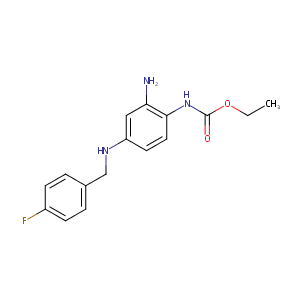
|
Download2D MOL |
|||
| Formula |
C16H18FN3O2
|
||||
| InChI |
InChI=1S/C16H18FN3O2/c1-2-22-16(21)20-15-8-7-13(9-14(15)18)19-10-11-3-5-12(17)6-4-11/h3-9,19H,2,10,18H2,1H3,(H,20,21)
|
||||
| InChIKey |
PCOBBVZJEWWZFR-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 150812-12-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
854071, 10239851, 12015005, 14800601, 24263039, 29302387, 49898865, 50233601, 53788352, 57339830, 58107328, 85174557, 85202062, 91615883, 92715048, 103220716, 103875964, 104032719, 104414526, 118046799, 118855330, 124490309, 125341609, 126623656, 126655065, 126665918, 128896436, 131299831, 134338628, 135077118, 135650896, 136345679, 136920410, 136946473, 137156938, 141970640, 152034730, 152159608, 160645840, 162011781, 162205078, 163123042, 163620909, 163686230, 164042455, 164194995, 164233343, 164786707, 174006313, 174527464
|
||||
| SuperDrug ATC ID |
N03AX21
|
||||
| Target and Pathway | |||||
| Target(s) | Potassium voltage-gated channel subfamily KQT member 3 | Target Info | Modulator | [531754], [531783], [531848] | |
| KEGG Pathway | Cholinergic synapse | ||||
| WikiPathways | Potassium Channels | ||||
| L1CAM interactions | |||||
| References | |||||
| Ref 536934 | Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX). Epilepsy Res. 2009 Jan;83(1):1-43. Epub 2008 Nov 12. | ||||
| Ref 539679 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2601). | ||||
| Ref 531754 | The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012 Mar;53(3):412-24. | ||||
| Ref 531783 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | ||||
| Ref 531848 | The urinary safety profile and secondary renal effects of retigabine (ezogabine): a first-in-class antiepileptic drug that targets KCNQ (K(v)7) potassium channels. Epilepsia. 2012 Apr;53(4):606-12. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

