Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01XLM
|
||||
| Former ID |
DAP000666
|
||||
| Drug Name |
Piperazine
|
||||
| Synonyms |
Anthalazine; Antiren; Diethylenediamine; Diethyleneimine; Dispermine; Eraverm; Hexahydropyrazine; Lumbrical; PZE; Piperazidine; Piperazin; Pipersol; Upixon; Uvilon; Vermex; Vermizine; Wurmirazin; Piperazin [German]; Piperazin [Germany]; Piperazine Dihydrochloride Dihydrochloride Hydrate; Piperazine [USAN]; Piperazine anhydrous; Piperazinium oleate; Pyrazine hexahydride; LTBB000432; Piperazine [UN2579] [Corrosive]; Eraverm (VAN); PIPERAZINE (HEXAHYDRATE); Piperazine (USP); Piperazine Hexa-Hydrate; Piperazine, anhydrous; Pyrazine, hexahy; Vermizine (TN); Worm-away; Asca-Trol No. 3; Worm-A-Ton; Hexahydro-1,4-diazine; 1,4-Diazacyclohexane; 1,4-Diethylenediamine; 1,4-Piperazine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antinematodal Agents
|
||||
| Company |
Glaxosmithkline
|
||||
| Structure |
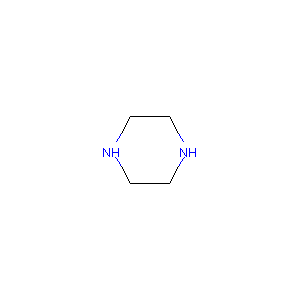
|
Download2D MOL |
|||
| Formula |
C4H10N2
|
||||
| Canonical SMILES |
C1CNCCN1
|
||||
| InChI |
1S/C4H10N2/c1-2-6-4-3-5-1/h5-6H,1-4H2
|
||||
| InChIKey |
GLUUGHFHXGJENI-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 110-85-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10174, 67380, 587321, 3134286, 5514467, 7847872, 8144317, 8149486, 8152972, 10503893, 11335213, 11360452, 11364007, 11366569, 11369131, 11377293, 11448501, 11448505, 11448541, 11448548, 11448550, 11448588, 11448590, 11448628, 11448668, 11448748, 11448779, 11448786, 11452303, 11452307, 11452335, 11452347, 11452377, 11452383, 11452387, 11452415, 11452425, 11452427, 11452455, 11452465, 11452467, 11452495, 11452505, 11452507, 11452535, 11452545, 11452575, 11452581, 11452617, 11452653
|
||||
| ChEBI ID |
ChEBI:28568
|
||||
| SuperDrug ATC ID |
P02CB01
|
||||
| SuperDrug CAS ID |
cas=000054911
|
||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor | Target Info | Antagonist | [535818] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

