Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03QKW
|
||||
| Former ID |
DIB003626
|
||||
| Drug Name |
KUC-7483
|
||||
| Synonyms |
Ritobegron ethyl ester; KUC-7322; Beta 3 adrenoceptor agonists (urinary incontinence), Kissei; N-phenylglycine derivatives, Kissei; 4-hydroxynorephedrine derivative (urinary incontinence), Kissei
|
||||
| Indication | Overactive bladder disorder [ICD9: 188, 596.51; ICD10:C67, N32.81] | Phase 1 | [524942] | ||
| Company |
Kissei Pharmaceutical Co Ltd
|
||||
| Structure |
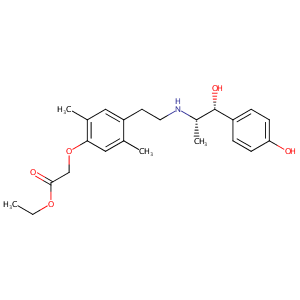
|
Download2D MOL |
|||
| Canonical SMILES |
c1(c(cc(c(c1)C)CCN[C@H]([C@@H](c1ccc(cc1)O)O)C)C)OCC(=O<br />)OCC
|
||||
| Target and Pathway | |||||
| Target(s) | Beta-3 adrenergic receptor | Target Info | Agonist | [531824], [531877] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

