Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Z4FE
|
||||
| Former ID |
DIB007601
|
||||
| Drug Name |
MIN-117
|
||||
| Indication | Major depressive disorder [ICD9: 296.2, 296.3, 710.0; ICD10:F32, F33, M32] | Phase 2 | [1] | ||
| Company |
Minerva neurosciences
|
||||
| Structure |
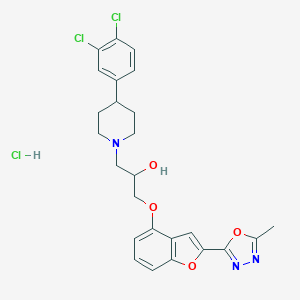
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 1A receptor | Target Info | Antagonist | [2] | |
| KEGG Pathway | cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Serotonergic synapse | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| 5HT1 type receptor mediated signaling pathway | |||||
| Reactome | Serotonin receptors | ||||
| G alpha (i) signalling events | |||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | ||||
| SIDS Susceptibility Pathways | |||||
| Monoamine GPCRs | |||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800037483) | ||||
| REF 2 | Company report (Minerva Neurosciences),MIN-101,Schizophrenia, 6 trials completed; Once a day formulation completed , Phase IIa completed; Phase IIb enrollment ongoing and expected to continue over the last 3 quarters of 2015. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

