Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T15739 | ||||
| Target Name | Tumor suppressor p53 | ||||
| Synonyms | Antigen NY-CO-13; P53; Phosphoprotein p53; Tumor suppressor p53; TP53 | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | TP53 | ||||
| Biochemical Class | Pore-forming pnc-27 peptide of 32 aas | ||||
| UniProt ID | P53_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Oral cancer | ||||
| Example drug | Contusugene ladenovec | Phase 3 | [1], [2] | ||
| Tissue | Oral tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.06 Z-score: 0.09 P-value: 7.01E-01 |
||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: -0.21 Z-score: -0.28 P-value: 6.48E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Acute myelocytic leukemia | ||||
| Example drug | ALT-801 | Phase 2 | [3], [2] | ||
| Tissue | Bone marrow | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.30 Z-score: 0.59 P-value: 7.90E-07 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Rectal cancer | ||||
| Example drug | ISA-P53-01 | Phase 1/2 | [4], [2] | ||
| Tissue | Rectal colon tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.20 Z-score: 0.87 P-value: 4.16E-01 |
||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.33 Z-score: 2.50 P-value: 4.87E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
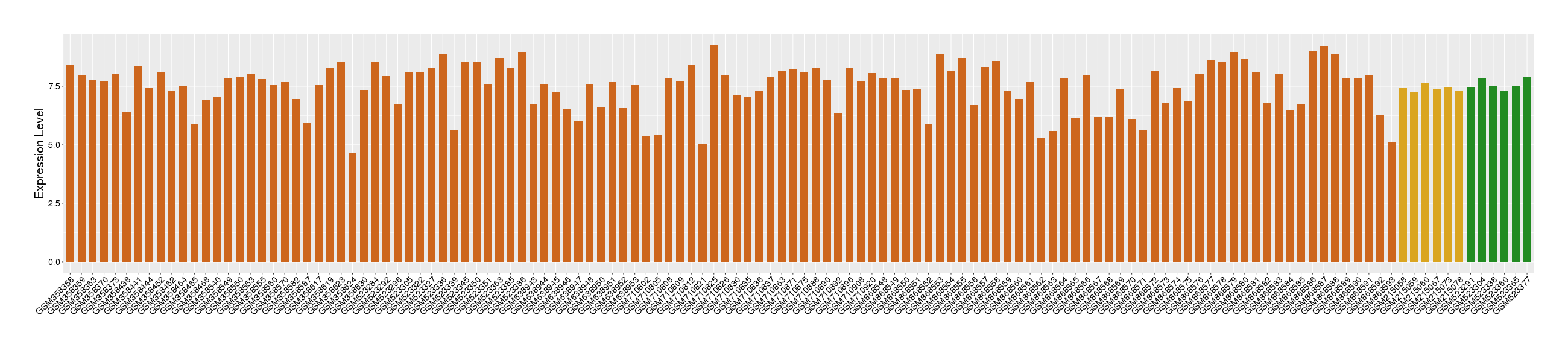
|
|||||
| Disease | Head and neck cancer | ||||
| Tissue | Head and neck tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.22 Z-score: 0.47 P-value: 1.22E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
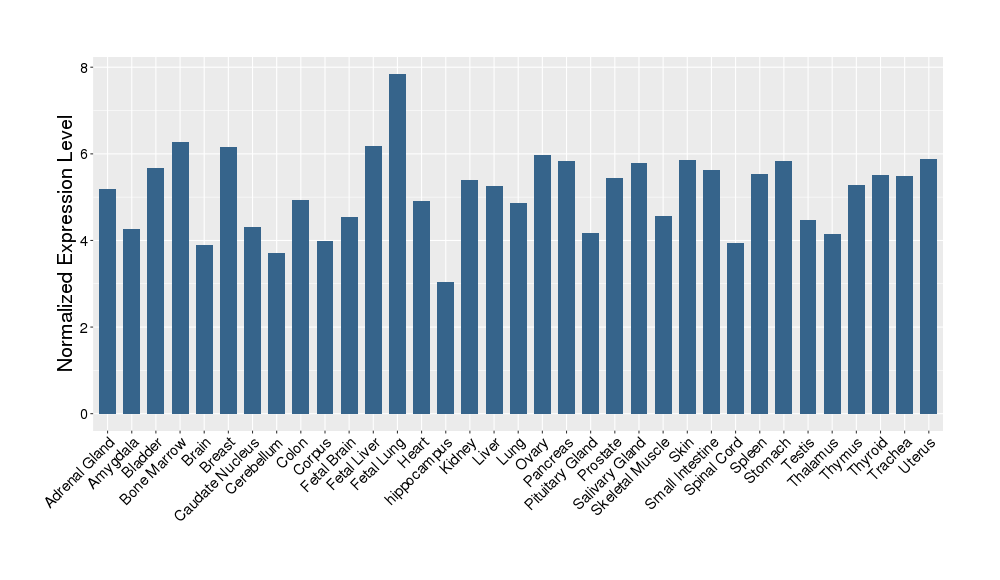
|
|||||
| Reference | |||||
| REF 1 | ClinicalTrials.gov (NCT00041613) Study to Compare the Overall Survival of Patients Receiving INGN 201 (Study Drug) With Patients Receiving Methotrexate. U.S. National Institutes of Health. | ||||
| REF 2 | NCBI GEO: archive for functional genomics data sets--update. | ||||
| REF 3 | Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011 Dec 15;17(24):7765-75. | ||||
| REF 4 | Clinical pipeline report, company report or official report of ISA Pharmaceuticals BV. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

