Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T61722 | ||||
| Target Name | G-protein coupled receptor 44 | ||||
| Synonyms | CD294; Chemoattractant receptor-homologous molecule expressed on Th2 cells; PTGDR2 | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | PTGDR2 | ||||
| Biochemical Class | GPCR rhodopsin | ||||
| UniProt ID | PD2R2_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Asthma | ||||
| Example drug | Setipiprant | Phase 3 | [1], [2] | ||
| Tissue | Nasal and bronchial airway | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.15 Z-score: -0.25 P-value: 6.11E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | AZD1981 | Phase 2 | [3], [4], [2] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 9.06E-03 Z-score: 0.05 P-value: 8.36E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
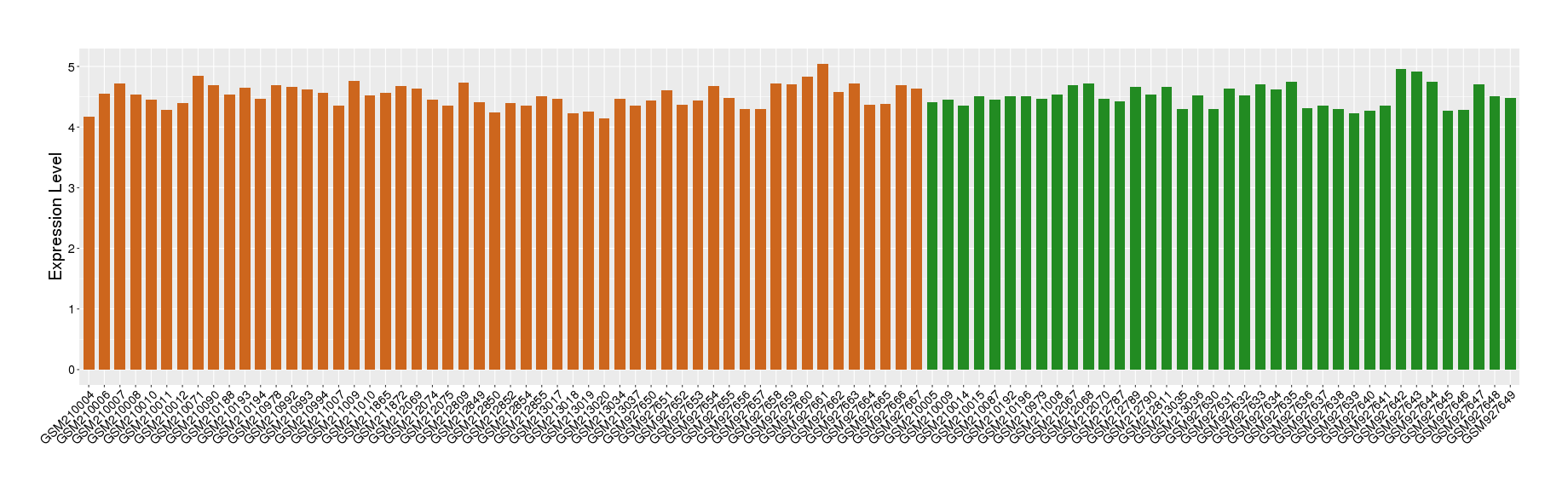
|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | AZD1981 | Phase 2 | [3], [4], [2] | ||
| Tissue | Small airway epithelium | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.14 Z-score: 0.78 P-value: 1.13E-03 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
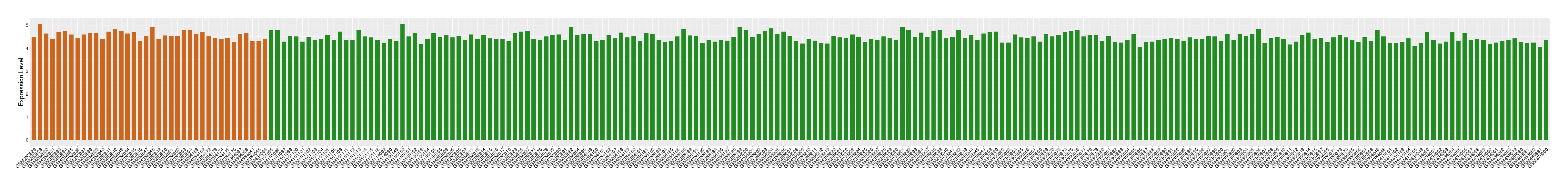
|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
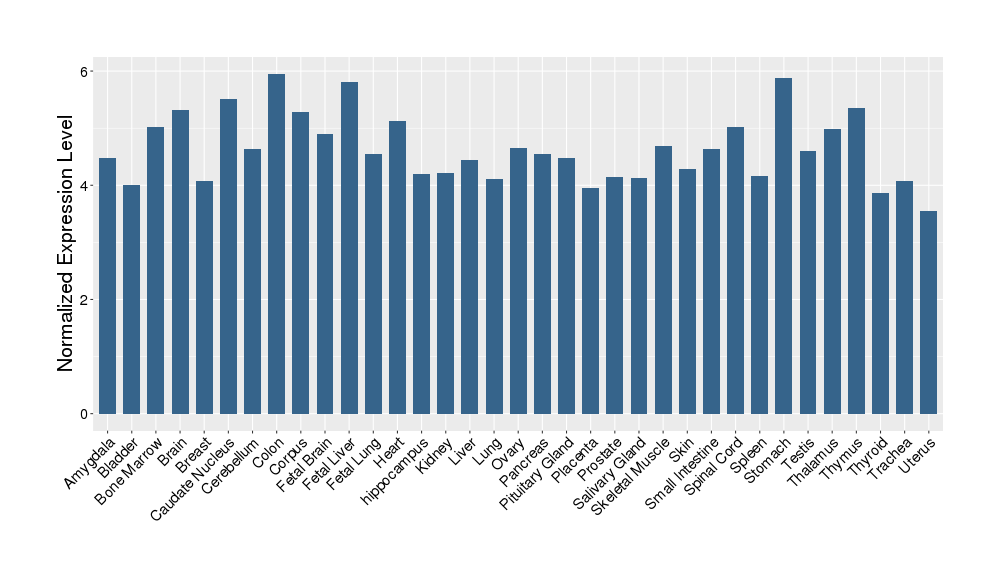
|
|||||
| Reference | |||||
| REF 1 | ClinicalTrials.gov (NCT01484119) Efficacy, Safety, and Tolerability Study of ACT-129968 in Patients With Seasonal Allergic Rhinitis. U.S. National Institutes of Health. | ||||
| REF 2 | NCBI GEO: archive for functional genomics data sets--update. | ||||
| REF 3 | ClinicalTrials.gov (NCT00766415) 14729-D9831C00002- 1 Month Biopsy Study. U.S. National Institutes of Health. | ||||
| REF 4 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7680). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

