Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T72841 | ||||
| Target Name | Steroid hormone receptor ERR1 | ||||
| Synonyms | ERR-alpha; ERRalpha; Estrogen receptor-like 1; Estrogen-relatedreceptor alpha; Estrogen-relatedreceptor, alpha; ESRRA | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | ESRRA | ||||
| Biochemical Class | Nuclear hormone receptor | ||||
| UniProt ID | ERR1_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
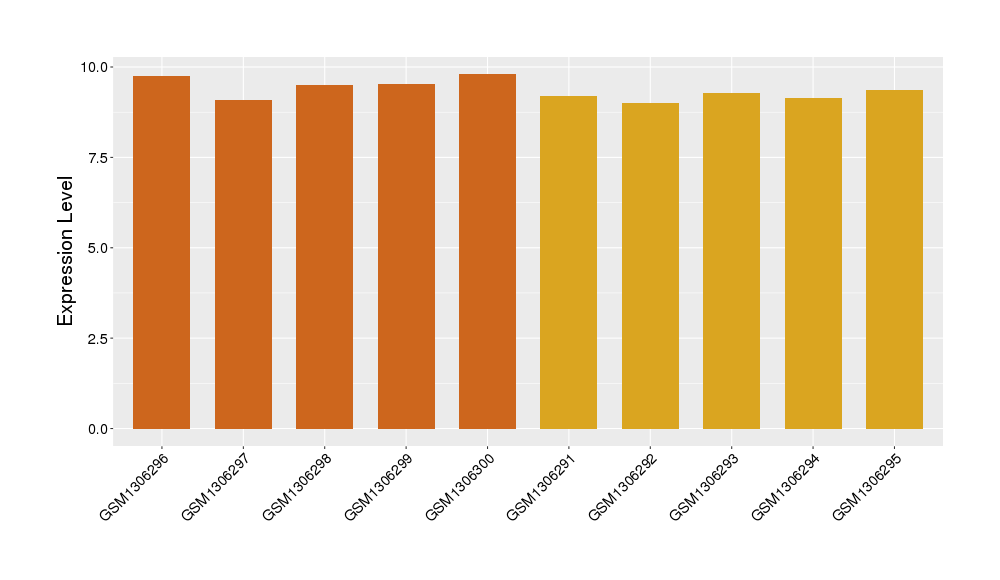
| Disease | Schizophrenia | ||||
| Example drug | Pregnenolone | Phase 4 | [1], [2], [3] | ||
| Tissue | Pre-frontal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 1.47E-02 Z-score: 0.06 P-value: 9.34E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
| Disease | Schizophrenia | ||||
| Example drug | Pregnenolone | Phase 4 | [1], [2], [3] | ||
| Tissue | Superior temporal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.07 Z-score: -0.62 P-value: 2.09E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Asthma | ||||
| Example drug | D-5519 | Phase 3 | [4], [3] | ||
| Tissue | Nasal and bronchial airway | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.26 Z-score: 0.47 P-value: 2.85E-08 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
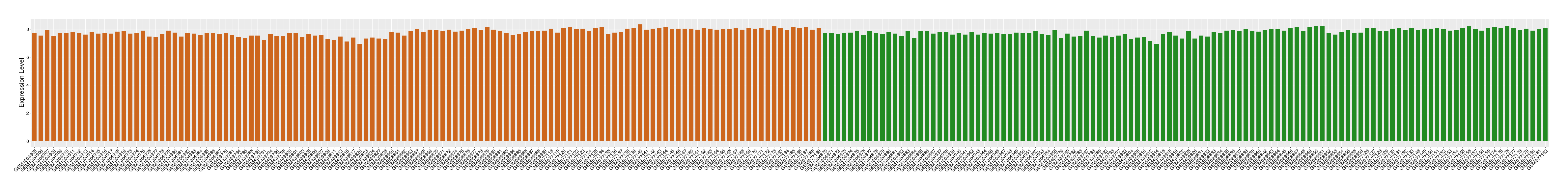
| Disease | Rheumatoid arthritis | ||||
| Example drug | AD-121 | Discontinued in Phase 1/2 | [5], [3] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.06 Z-score: 0.14 P-value: 3.46E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
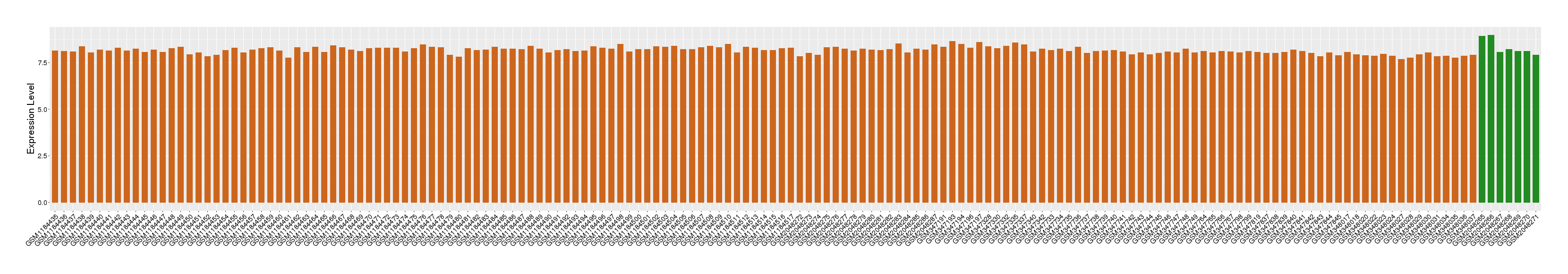
| Disease | Atopic dermatitis | ||||
| Tissue | Skin | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.51 Z-score: 3.32 P-value: 6.95E-14 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
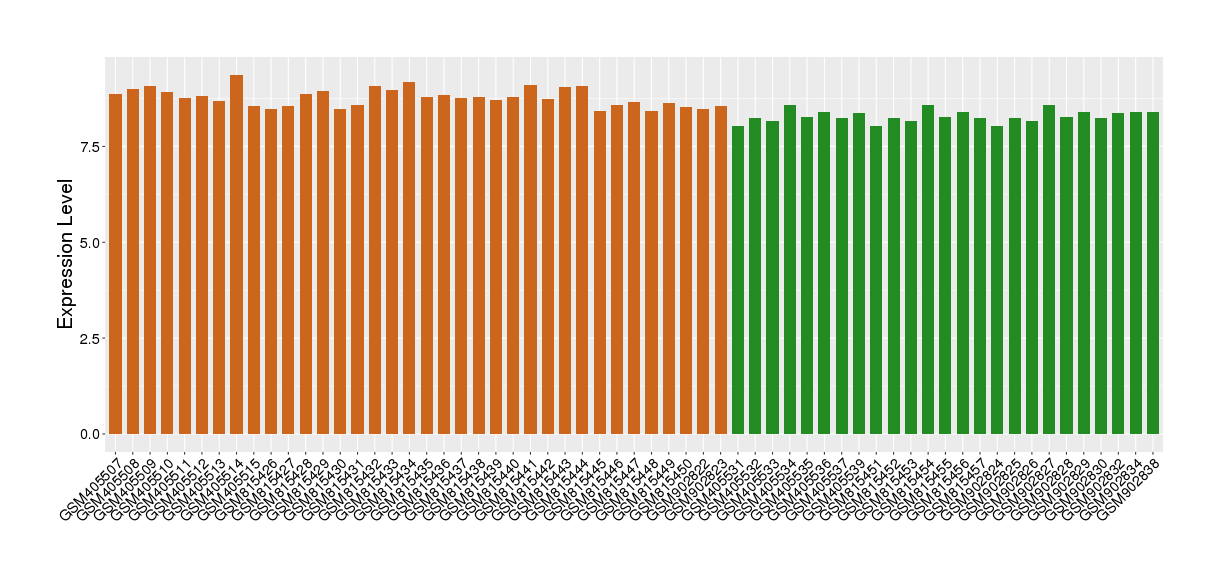
| Disease | Eosinophilic gastritis | ||||
| Tissue | Gastric antrum tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.34 Z-score: 2.61 P-value: 5.63E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
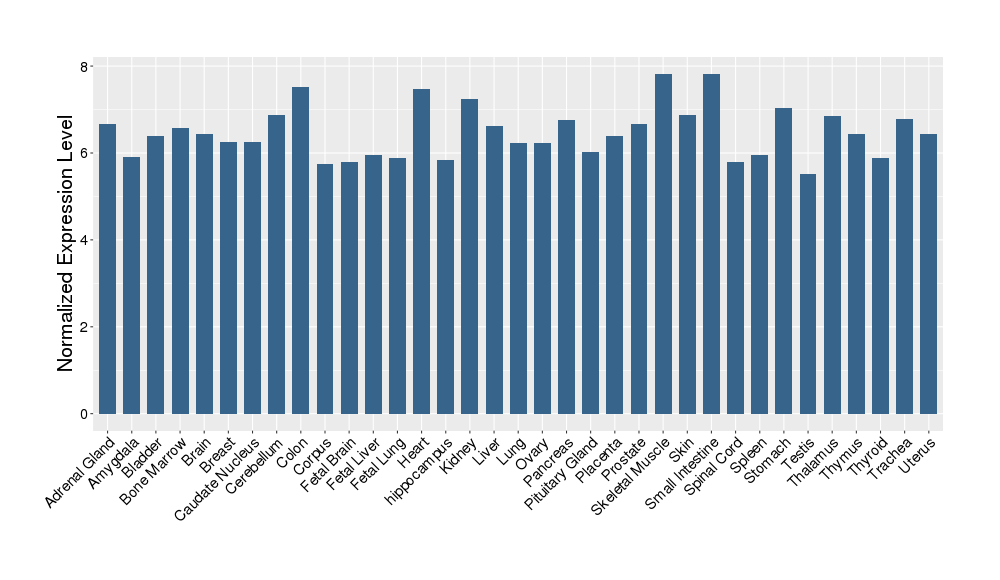
|
|||||
| Reference | |||||
| REF 1 | ClinicalTrials.gov (NCT01409096) Study Using Pregnenolone to Treat Bipolar Depression. U.S. National Institutes of Health. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2376). | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. | ||||
| REF 4 | ClinicalTrials.gov (NCT02270450) S1316, Surgery or Non-Surgical Management in Treating Patients With Intra-Abdominal Cancer and Bowel Obstruction. U.S. National Institutes of Health. | ||||
| REF 5 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015041) | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

