Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T79160 | ||||
| Target Name | Glial cell neurotrophic factor ligand | ||||
| Synonyms | ATF; Astrocytederived trophic factor; Glial cell linederived neurotrophic factor; hGDNF; GDNF | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | GDNF | ||||
| Biochemical Class | Transforming growth factor | ||||
| UniProt ID | GDNF_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Lateral sclerosis | ||||
| Example drug | Liatermin | Phase 1/2 | [1], [2] | ||
| Tissue | Cervical spinal cord | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.08 Z-score: 0.31 P-value: 2.29E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
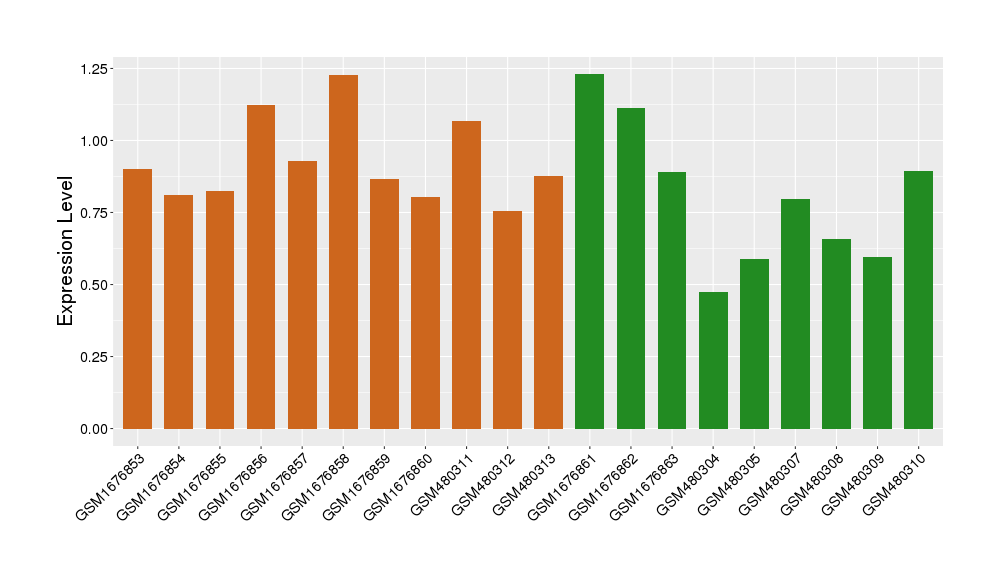
|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
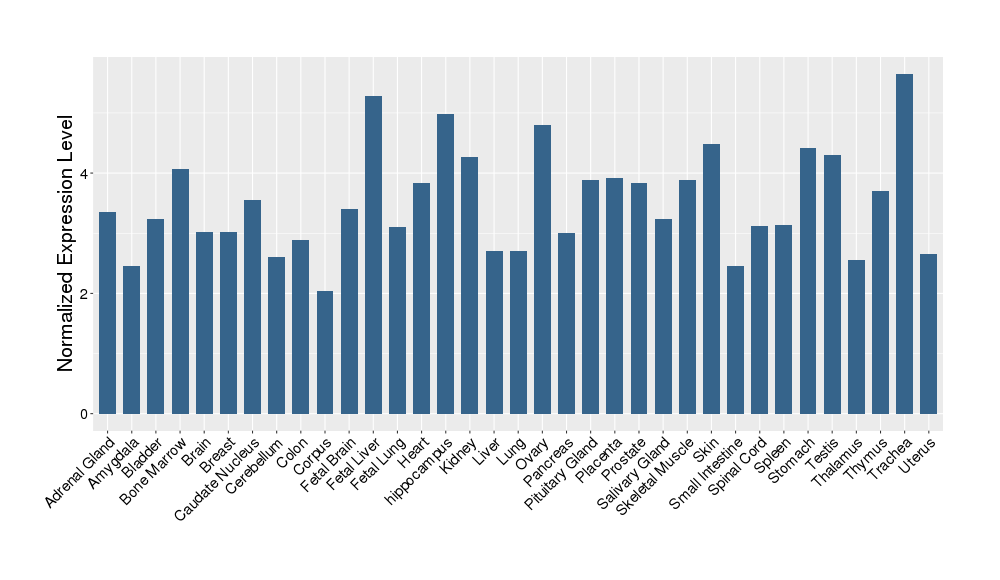
|
|||||
| Reference | |||||
| REF 1 | ClinicalTrials.gov (NCT00111982) Trial of Extended Treatment With Liatermin (r-metHuGDNF) Administered by Continuous Intraputaminal (IPu)Infusion to Subjects With Idiopathic Parkinson's Disease Who Have Completed a Previous Trial of Liatermin. U.S. National Institutes of Health. | ||||
| REF 2 | NCBI GEO: archive for functional genomics data sets--update. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

