Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01BBR
|
||||
| Former ID |
DIB004273
|
||||
| Drug Name |
Dezinamide
|
||||
| Synonyms |
ADD-94057; ADDR-84116; AHR-11748; AN-051
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Epileptic seizures [ICD9: 345.9, 780.3; ICD10:G40, P90, R56] | Discontinued in Phase 2 | [1] | ||
| Company |
A H Robins Co Inc
|
||||
| Structure |
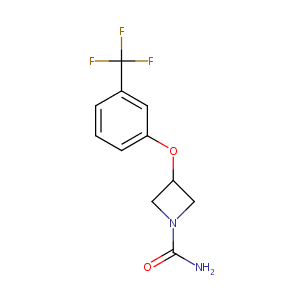
|
Download2D MOL |
|||
| Formula |
C11H11F3N2O2
|
||||
| Canonical SMILES |
N1(C(=O)N)CC(C1)Oc1cc(C(F)(F)F)ccc1
|
||||
| CAS Number |
CAS 91077-32-6
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor | Target Info | Modulator | [2] | |
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000082) | ||||
| REF 2 | Clobazam, oxcarbazepine, tiagabine, topiramate, and other new antiepileptic drugs. Epilepsia. 1995;36 Suppl 2:S105-14. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

