Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03GAX
|
||||
| Former ID |
DIB000037
|
||||
| Drug Name |
TAK-802
|
||||
| Indication | Urinary dysfunction [ICD10:N39.3-N39.4] | Discontinued in Phase 2 | [547886] | ||
| Company |
Takeda Pharmaceutical Co Ltd
|
||||
| Structure |
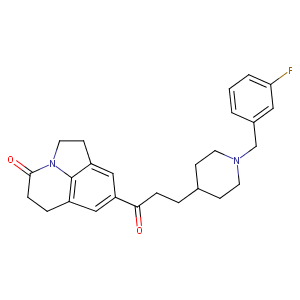
|
Download2D MOL |
|||
| Canonical SMILES |
N12c3c(cc(cc3CCC1=O)C(=O)CCC1CCN(Cc3cc(F)ccc3)CC1)CC2
|
||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [526952] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

