Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04LOR
|
||||
| Former ID |
DIB015859
|
||||
| Drug Name |
BMS-919373
|
||||
| Indication | Atrial fibrillation [ICD9: 272, 427.31; ICD10:E78, I48] | Phase 2 | [1] | ||
| Company |
Bristol-myers squibb
|
||||
| Structure |
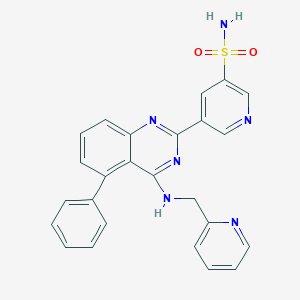
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Potassium voltage-gated channel subfamily A member 5 | Target Info | Inhibitor | [2] | |
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| Reactome | Voltage gated Potassium channels | ||||
| WikiPathways | Potassium Channels | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02156076) A Blinded Study to Evaluate Effect on Atrial Fibrillation Burden in Patients With Paroxysmal Atrial Fibrillation. U.S. National Institutes of Health. | ||||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035504) | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

