Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04ZWQ
|
||||
| Former ID |
DIB007011
|
||||
| Drug Name |
Org-25435
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Epilepsy [ICD10:G40] | Phase 1 | [1] | ||
| Company |
Organon BioSciences
|
||||
| Structure |
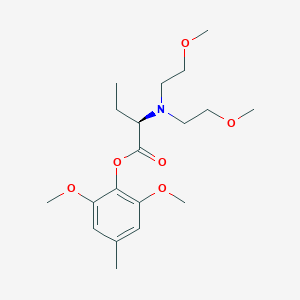
|
Download2D MOL |
|||
| Formula |
C19H31NO6
|
||||
| Canonical SMILES |
c1(OC(=O)[C@H](N(CCOC)CCOC)CC)c(cc(cc1OC)C)OC
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | GABA A receptor | Target Info | Modulator | [2] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | |||||
| GABAergic synapse | |||||
| Morphine addiction | |||||
| Nicotine addiction | |||||
| Reactome | Ligand-gated ion channel transport | ||||
| GABA A receptor activation | |||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transport | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01062867) First Administration to Man Of Org 25435 a New Intravenous Anesthetic. U.S. National Institutes of Health. | ||||
| REF 2 | First administration to man of Org 25435, an intravenous anaesthetic: A Phase 1 Clinical Trial. BMC Anesthesiol. 2010 Jun 29;10:10. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

