Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05LRD
|
||||
| Former ID |
DNCL001932
|
||||
| Drug Name |
BMS-933043
|
||||
| Indication | Psychiatric disorder [ICD9: 290-319; ICD10:F01-F99] | Phase 1 | [1] | ||
| Company |
Bristol-Myers Squibb
|
||||
| Structure |
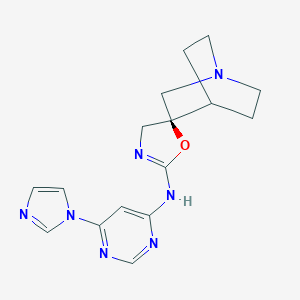
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Neuronal acetylcholine receptor protein, alpha-7 chain | Target Info | Modulator | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Cholinergic synapse | |||||
| Nicotine addiction | |||||
| Chemical carcinogenesis | |||||
| PANTHER Pathway | Alzheimer disease-amyloid secretase pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Reactome | Highly calcium permeable postsynaptic nicotinic acetylcholine receptors | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01605994) Multiple Ascending-Dose Study to Evaluate the Safety, Tolerability and Pharmacokinetics of BMS-933043 in Healthy Subjects. U.S. National Institutes of Health. | ||||
| REF 2 | Clinical pipeline report, company report or official report of Phrma.org Alzheimers 2012. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

