Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07QAR
|
||||
| Former ID |
DCL000512
|
||||
| Drug Name |
Dalbavancin
|
||||
| Synonyms |
Zeven; Zeven (TN); Dalbavancin (USAN/INN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Pfizer
|
||||
| Structure |
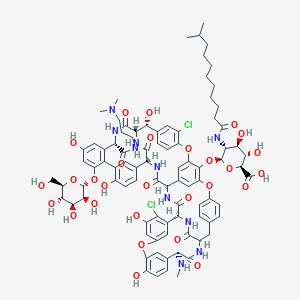
|
Download2D MOL |
|||
| Formula |
C88H100Cl2N10O28
|
||||
| Canonical SMILES |
CC(C)CCCCCCCCC(=O)NC1C(C(C(OC1OC2=C3C=C4C=C2OC5=C(C=C(C<br />=C5)C(C6C(=O)NC(C7=CC(=CC(=C7C8=C(C=CC(=C8)C(C(=O)N6)NC<br />(=O)C4NC(=O)C9C1=CC(=CC(=C1Cl)O)OC1=C(C=CC(=C1)C(C(=O)N<br />C(CC1=CC=C(O3)C=C1)C(=O)N9)NC)O)O)OC1C(C(C(C(O1)CO)O)O)<br />O)O)C(=O)NCCCN(C)C)O)Cl)C(=O)O)O)O
|
||||
| InChI |
1S/C88H100Cl2N10O28/c1-38(2)13-10-8-6-7-9-11-14-61(106)94-70-73(109)75(111)78(86(120)121)128-87(70)127-77-58-31-43-32-59(77)124-55-24-19-42(29-50(55)89)71(107)69-85(119)98-67(80(114)92-25-12-26-100(4)5)48-33-44(102)34-57(125-88-76(112)74(110)72(108)60(37-101)126-88)62(48)47-28-40(17-22-52(47)103)65(82(116)99-69)95-83(117)66(43)96-84(118)68-49-35-46(36-54(105)63(49)90)123-56-30-41(18-23-53(56)104)64(91-3)81(115)93-51(79(113)97-68)27-39-15-20-45(122-58)21-16-39/h15-24,28-36,38,51,60,64-76,78,87-88,91,101-105,107-112H,6-14,25-27,37H2,1-5H3,(H,92,114)(H,93,115)(H,94,106)(H,95,117)(H,96,118)(H,97,113)(H,98,119)(H,99,116)(H,120,121)/t51-,60-,64-,65-,66-,67?,68+,69+,70-,71-,72-,73-,74+,75+,76+,78+,87-,88+/m1/s1
|
||||
| InChIKey |
KGPGQDLTDHGEGT-VBZOGQDBSA-N
|
||||
| CAS Number |
CAS 91097-81-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
J01XA04
|
||||
| Target and Pathway | |||||
| Target(s) | Triacylglycerol lipase, pancreatic | Target Info | Inhibitor | [536589], [549974] | |
| BioCyc Pathway | Triacylglycerol degradation | ||||
| Retinol biosynthesis | |||||
| Reactome | Retinoid metabolism and transport | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

