| Drug General Information |
| Drug ID |
D07VBA
|
| Drug Name |
Estradiol Valerate
|
| Synonyms |
Delestrogen; Estradiol Valerate
|
| Drug Type |
Small molecular drug
|
| Indication |
Hormone replacement therapy [ICD9: V07.4; ICD10:Z79.890]
|
Approved |
[1]
|
|---|
| Company |
Par Sterile Products Llc
|
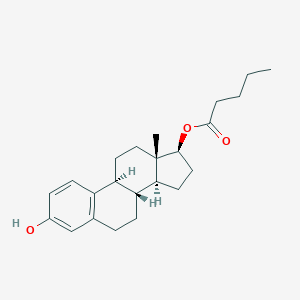
| Structure |
|
Download
2D MOL
3D MOL
|
| Formula |
C23H32O3
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Estrogen receptor |
Target Info |
Modulator |
[2]
|
|---|
|
KEGG Pathway
|
Estrogen signaling pathway
|
|
Prolactin signaling pathway
|
|
Thyroid hormone signaling pathway
|
|
Endocrine and other factor-regulated calcium reabsorption
|
|
Proteoglycans in cancer
|
|
NetPath Pathway
|
FSH Signaling Pathway
|
|
EGFR1 Signaling Pathway
|
|
RANKL Signaling Pathway
|
|
Pathway Interaction Database
|
Regulation of nuclear SMAD2/3 signaling
|
|
Signaling events mediated by HDAC Class II
|
|
Plasma membrane estrogen receptor signaling
|
|
LKB1 signaling events
|
|
Regulation of Telomerase
|
|
ATF-2 transcription factor network
|
|
AP-1 transcription factor network
|
|
FOXM1 transcription factor network
|
|
Validated nuclear estrogen receptor alpha network
|
|
Signaling mediated by p38-alpha and p38-beta
|
|
FOXA1 transcription factor network
|
|
Reactome
|
Nuclear signaling by ERBB4
|
|
Nuclear Receptor transcription pathway
|
|
WikiPathways
|
Estrogen signaling pathway
|
|
Nuclear Receptors Meta-Pathway
|
|
Estrogen Receptor Pathway
|
|
Signaling by ERBB4
|
|
JAK/STAT
|
|
Integrated Pancreatic Cancer Pathway
|
|
Leptin signaling pathway
|
|
miR-targeted genes in muscle cell - TarBase
|
|
Integrated Breast Cancer Pathway
|
|
Nuclear Receptors
|
| References |
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 |
|---|
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. |
|---|