Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08CGU
|
||||
| Former ID |
DCL000496
|
||||
| Drug Name |
Bevacizumab + Rituximab
|
||||
| Synonyms |
Rituxan (TN)
|
||||
| Drug Type |
Antibody
|
||||
| Indication | Lymphoma; Non-hodgkin's lymphoma [ICD9: 200, 202, 202.8; ICD10:C81-C86, C82-C85] | Phase 3 | [522045] | ||
| Company |
Roche
|
||||
| Structure |
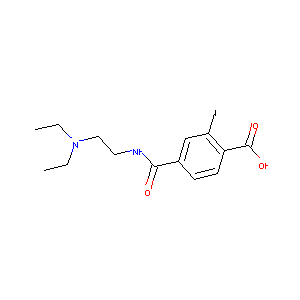
|
Download2D MOL |
|||
| Formula |
C14H18ILiN2O3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | B-lymphocyte antigen CD20 | Target Info | [551607] | ||
| Vascular endothelial growth factor | Target Info | Inhibitor | [551607] | ||
| KEGG Pathway | Hematopoietic cell lineage | ||||
| References | |||||
| Ref 522045 | ClinicalTrials.gov (NCT00486759) A Study of Bevacizumab (Avastin) in Combination With Rituximab (MabThera) and CHOP (Cyclophosphamide, Hydroxydaunorubicin [Doxorubicin], Oncovin [Vincristine], Prednisone) Chemotherapy in Patients With Diffuse Large B-cell Lymphoma. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

