Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08USJ
|
||||
| Former ID |
DAP000563
|
||||
| Drug Name |
Neostigmine
|
||||
| Synonyms |
Eustigmin; Eustigmine; Intrastigmina; Juvastigmin; Neostigmin; Neostigminum; Prostigmin; Prostigmine; Prozerin; Sulfonatooxymethane; Synstigmin; Syntostigmine; Vagostigmin; Vagostigmine; Neostigmine [BAN]; Neostigmine omega; M-Trimethylammoniumphenyldimethylcarbamate; Neostigmine (BAN); Prostigmin (TN); Vagostigmin (TN); [3-(dimethylcarbamoyloxy)phenyl]-trimethylazanium; [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium; [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium bromide; Ammonium, (m-hydroxyphenyl)trimethyl-, dimethylcarbamate (ester); Benzenaminium, 3-(((dimethylamino)carbonyl)oxy)-N,N,N-trimethyl-(9CI); (m-Hydroxyphenyl)trimethylammonium dimethylcarbamate; (m-Hydroxyphenyl)trimethylammonium dimethylcarbamate (ester); 3-Trimethylammoniumphenyl N,N-dimethylcarbamate; 3-[(dimethylcarbamoyl)oxy]-N,N,N-trimethylanilinium; 3-{[(dimethylamino)carbonyl]oxy}-N,N,N-trimethylanilinium
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Myasthenia gravis [ICD9: 358; ICD10:G70.0] | Approved | [550269] | ||
| Therapeutic Class |
Parasympathomimetics
|
||||
| Structure |
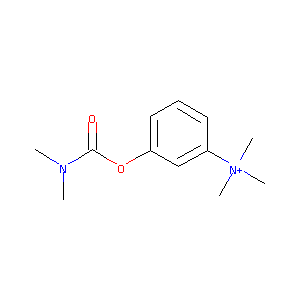
|
Download2D MOL |
|||
| Formula |
C12H19N2O2+
|
||||
| Canonical SMILES |
CN(C)C(=O)OC1=CC=CC(=C1)[N+](C)(C)C
|
||||
| InChI |
1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1
|
||||
| InChIKey |
ALWKGYPQUAPLQC-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 59-99-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9467, 866380, 4398035, 4635954, 7980106, 8152746, 11111527, 11111528, 11335340, 11335373, 11360579, 11360612, 11363955, 11366517, 11369079, 11373762, 11377241, 11461551, 11461584, 11466496, 11467616, 11485012, 11486251, 11489094, 11491887, 11494875, 15121488, 26756597, 29223551, 46509161, 47216650, 47291007, 47365046, 47588860, 47736334, 47736335, 47810618, 48110324, 48416319, 49698982, 49868416, 50100289, 50100290, 50103950, 50765645, 53786926, 57322279, 81044623, 85787484, 87691541
|
||||
| SuperDrug ATC ID |
N07AA01; S01EB06
|
||||
| SuperDrug CAS ID |
cas=000059994
|
||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [537124], [537383] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| References | |||||
| Ref 537124 | Neuromuscular blockade, reversal agent use, and operating room time: retrospective analysis of US inpatient surgeries. Curr Med Res Opin. 2009 Apr;25(4):943-50. | ||||
| Ref 537383 | Screening of acetylcholinesterase inhibitors by CE after enzymatic reaction at capillary inlet. J Sep Sci. 2009 May;32(10):1748-56. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

