Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08ZRR
|
||||
| Former ID |
DIB006016
|
||||
| Drug Name |
KW-5092
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89] | Phase 1 | [534378] | ||
| Structure |
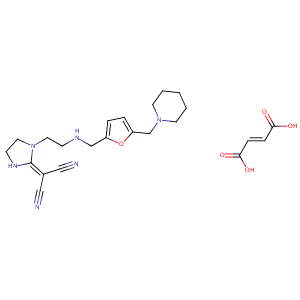
|
Download2D MOL |
|||
| Formula |
C23H30N6O5
|
||||
| CAS Number |
CAS 135017-30-0
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [534378] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

