Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0D4CY
|
||||
| Former ID |
DAP001200
|
||||
| Drug Name |
Sulfapyridine
|
||||
| Synonyms |
Adiplon; Coccoclase; Dagenan; Eubasin; Eubasinum; Haptocil; Piridazol; Plurazol; Pyriamid; Pyridazol; Relbapiridina; Ronin;Septipulmon; Solfapiridina; Streptosilpyridine; Sulfapiridina; Sulfapyridinum; Sulfidin; Sulfidine; Sulphapyridin; Sulphapyridine; Thioseptal; Trianon; Solfapiridina [DCIT]; M and B 693; A-499; ALBB-006215; M + B 693; M&B 693; M+B 693; Sulfapiridina [INN-Spanish]; Sulfapyridine (TN); Sulfapyridinum [INN-Latin]; AO-801/41077453; N(1)-Pyridylsulfanilamide; N(sup1)-Pyridylsulfanilamide; N-2-Pyridylsulfanilamide; N1-2-Pyridylsulfanilamide; Sulfapyridine (USP/INN); Sulfapyridine [USAN:INN:BAN]; N'-2-Pyridylsulfanilide; N(1)-2-Pyridylsulfanilamide; N(sup 1)-2-Pyridylsulfanilamide; N1-(Pyridin-2-yl)sulfanilamide; Sulfanilamide, N1-2-pyridyl-(8CI); 2-(4-Aminobenzenesulfonamido)pyridine; 2-(p-Aminobenzenesulphonamido)pyridine; 2-Sulfanilamidopyridin; 2-Sulfanilamidopyridin [German]; 2-Sulfanilamidopyridine; 2-Sulfanilyl aminopyridine; 2-Sulfanilylaminopyridine; 2-Sulfapyridine; 4-(2-Pyridinylsulfonyl)aniline; 4-AMINO-N-2-PYRIDINYLBENZENESULFONAMIDE; 4-Amino-N,2-pyridinylbenzenesulfonamide; 4-Amino-N-2-pyridinyl-benzenesulfonamide; 4-Amino-N-[2-pyridyl]benzene sulfonamide; 4-[(2-Pyridylamino)sulfonyl]aniline; 4-amino-N-(pyridin-2-yl)benzenesulfonamide; 4-amino-N-pyridin-2-yl-benzenesulfonamide; 4-amino-N-pyridin-2-ylbenzenesulfonamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Dermatologic Agents
|
||||
| Structure |
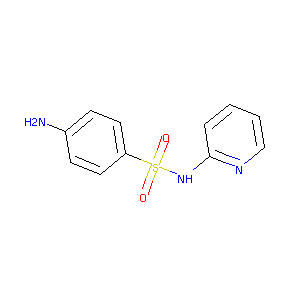
|
Download2D MOL |
|||
| Formula |
C11H11N3O2S
|
||||
| Canonical SMILES |
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N
|
||||
| InChI |
1S/C11H11N3O2S/c12-9-4-6-10(7-5-9)17(15,16)14-11-3-1-2-8-13-11/h1-8H,12H2,(H,13,14)
|
||||
| InChIKey |
GECHUMIMRBOMGK-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 144-83-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
70988, 96525, 603199, 855656, 866442, 5631195, 7820105, 7849492, 7980704, 8149538, 8153278, 10321924, 10508192, 11112302, 11335391, 11360630, 11363890, 11366452, 11369014, 11372864, 11373890, 11377176, 11461602, 11466790, 11467910, 11485181, 11486479, 11489139, 11491588, 11492161, 11494810, 15221159, 17140599, 24899692, 26611930, 26679786, 26746948, 29224390, 46506991, 47291019, 47365061, 47365062, 47515199, 47662144, 47736350, 48110333, 48110334, 48424128, 49698734, 49855022
|
||||
| SuperDrug ATC ID |
J01EB04
|
||||
| SuperDrug CAS ID |
cas=000144832
|
||||
| Target and Pathway | |||||
| Target(s) | Dihydropteroate synthetase | Target Info | Inhibitor | [537591] | |
| References | |||||
| Ref 537591 | A confirmatory method for the simultaneous extraction, separation, identification and quantification of Tetracycline, Sulphonamide, Trimethoprim and Dapsone residues in muscle by ultra-high-performance liquid chromatography-tandem mass spectrometry according to Commission Decision 2002/657/EC. J Chromatogr A. 2009 Jun 9. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

