Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0L4JT
|
||||
| Former ID |
DAP001193
|
||||
| Drug Name |
Sulfanilamide
|
||||
| Synonyms |
AAS; AVC; Albexan; Albosal; Ambeside; Antistrept; Astreptine; Astrocid; Bacteramid; Bactesid; Collomide; Colsulanyde; Copticide; Deseptyl; Desseptyl; Dipron; Ergaseptine; Erysipan; Estreptocida; Exoseptoplix; Gerison; Gombardol; Infepan; Lusil; Lysococcine; Neococcyl; Orgaseptine; PABS; Prontalbin; Prontylin; Proseptal; Proseptine; Proseptol; Pysococcine; SAN; Sanamid; Septanilam; Septinal; Septolix; Septoplex; Septoplix; Solfanilamide; Stramid; Strepamide; Strepsan; Streptagol; Streptamid; Streptamin; Streptasol; Streptocid; Streptocide; Streptocidum; Streptoclase; Streptocom; Streptol; Strepton; Streptopan; Streptosil; Streptozol; Streptozone; Streptrocide; Sulfamidyl; Sulfamine; Sulfana; Sulfanalone; Sulfanidyl; Sulfanil; Sulfanilamida; Sulfanilamidum; Sulfocidin; Sulfocidine; Sulfonamide; Sulfonylamide; Sulphanilamide; Sulphonamide; Therapol; Tolder; Prontosil Album; Prontosil I; Prontosil White; Pronzin Album; Rubiazol A; Septamide Album; Solfanilamide [DCIT]; Stopton Album; Streptocid album; Streptocide White; Sulfanilamide Vaginal Cream; Sulfanilimidic acid; Sulfonamide P; Sulphanilamide Extra Pure; Sulphanilamide Gr; White streptocide; F 1162; Fourneau 1162; Ro13354; A-349; AVC (TN); Aromatic/heteroaromatic sulfonamide 2; F-1162; P-Aminobenzenesulfamide; P-Aminobenzenesulfonamide; P-Aminobenzenesulfonylamide; P-Aminobenzensulfonamide; P-Aminophenylsulfonamide; P-Anilinesulfonamide; P-Sulfamidoaniline; P-Sulfamoylaniline; Streptocid (TN); Streptocide (VAN); Sulfanilamida [INN-Spanish]; Sulfanilamide (INN); Sulfanilamide [INN:DCF]; Sulfanilamidum [INN-Latin]; Aniline-p-sulfonic amide; I.C. 56; BENZENESULFONIC ACID,4-AMINO,AMIDE SULFANILAMIDE; N4,N4-Bis(2-bromoethyl)sulfanilamide;N(sup 4),N(sup 4)-Bis(2-bromoethyl)sulfanilamide; N(sup4),N(sup4)-Bis(2-bromoethyl)sulfanilamide; Benzenesulfonamide, 4-(bis(2-bromoethyl)amino)-(9CI); Sulfanilamide, N4,N4-bis(2-bromoethyl)-(8CI); 1162 F; 4-(Bis(2-bromoethyl)amino)benzenesulfonamide; 4-Aminobenzene-1-Sulfonamide; 4-Aminobenzenesulfonamide; 4-Aminophenylsulfonamide; 4-Sulfamoylaniline; 4-[bis(2-bromoethyl)amino]benzenesulfonamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [538372] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Structure |
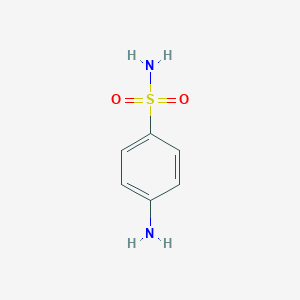
|
Download2D MOL |
|||
| Formula |
C10H14Br2N2O2S
|
||||
| Canonical SMILES |
C1=CC(=CC=C1N(CCBr)CCBr)S(=O)(=O)N
|
||||
| InChI |
1S/C10H14Br2N2O2S/c11-5-7-14(8-6-12)9-1-3-10(4-2-9)17(13,15)16/h1-4H,5-8H2,(H2,13,15,16)
|
||||
| InChIKey |
WWKINABOMDPLIE-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 63-74-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| ChEBI ID |
ChEBI:45373
|
||||
| SuperDrug ATC ID |
D06BA05; J01EB06
|
||||
| SuperDrug CAS ID |
cas=000063741
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Dihydropteroate synthetase | Target Info | Modulator | [556264] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

