Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M8OC
|
||||
| Former ID |
DNCL003065
|
||||
| Drug Name |
Bavisant
|
||||
| Synonyms |
JNJ-31001074
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Attention deficit hyperactivity disorder [ICD9: 314; ICD10:F90] | Phase 2 | [1] | ||
| Company |
Johnson & Johnson Pharmaceutical Research & Development
|
||||
| Structure |
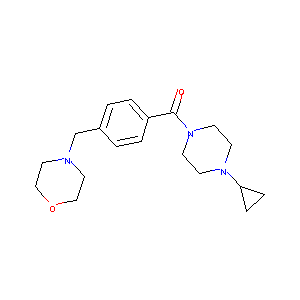
|
Download2D MOL |
|||
| Formula |
C19H27N3O2
|
||||
| Canonical SMILES |
C1CC1N2CCN(CC2)C(=O)C3=CC=C(C=C3)CN4CCOCC4
|
||||
| InChI |
1S/C19H27N3O2/c23-19(22-9-7-21(8-10-22)18-5-6-18)17-3-1-16(2-4-17)15-20-11-13-24-14-12-20/h1-4,18H,5-15H2
|
||||
| InChIKey |
BGBVSGSIXIIREO-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Histamine H3 receptor | Target Info | Antagonist | [2] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Reactome | Histamine receptors | ||||
| G alpha (i) signalling events | |||||
| WikiPathways | Monoamine Transport | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00566449) A Safety and Effectiveness Study of JNJ-31001074 in Adults With Attention-Deficit/Hyperactivity Disorder.. U.S. National Institutes of Health. | ||||
| REF 2 | Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012 May 1;26(5):421-34. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

