Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0MF7U
|
||||
| Former ID |
DNC013467
|
||||
| Drug Name |
PF-3052334
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Atherosclerosis [ICD9: 414.0, 440; ICD10:I70] | Discontinued in Phase 1 | [1] | ||
| Structure |
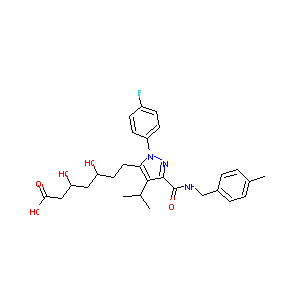
|
Download2D MOL |
|||
| Formula |
C28H33FN3NaO5
|
||||
| Canonical SMILES |
CC1=CC=C(C=C1)CNC(=O)C2=NN(C(=C2C(C)C)CCC(CC(CC(=O)[O-]<br />)O)O)C3=CC=C(C=C3)F.[Na+]
|
||||
| InChI |
1S/C28H34FN3O5.Na/c1-17(2)26-24(13-12-22(33)14-23(34)15-25(35)36)32(21-10-8-20(29)9-11-21)31-27(26)28(37)30-16-19-6-4-18(3)5-7-19;/h4-11,17,22-23,33-34H,12-16H2,1-3H3,(H,30,37)(H,35,36);/q;+1/p-1/t22-,23-;/m1./s1
|
||||
| InChIKey |
VEGKDAAILHTMLD-OHIDFYLOSA-M
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | Target Info | Inhibitor | [2] | |
| BioCyc Pathway | Superpathway of geranylgeranyldiphosphate biosynthesis I (via mevalonate) | ||||
| Superpathway of cholesterol biosynthesis | |||||
| Mevalonate pathway | |||||
| KEGG Pathway | Terpenoid backbone biosynthesis | ||||
| Metabolic pathways | |||||
| Biosynthesis of antibiotics | |||||
| AMPK signaling pathway | |||||
| Bile secretion | |||||
| NetPath Pathway | IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| TSH Signaling Pathway | |||||
| PANTHER Pathway | Cholesterol biosynthesis | ||||
| PathWhiz Pathway | Steroid Biosynthesis | ||||
| WikiPathways | Statin Pathway | ||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | |||||
| Activation of Gene Expression by SREBP (SREBF) | |||||
| SREBF and miR33 in cholesterol and lipid homeostasis | |||||
| Integrated Breast Cancer Pathway | |||||
| SREBP signalling | |||||
| Cholesterol Biosynthesis | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025539) | ||||
| REF 2 | J Med Chem. 2008 Jan 10;51(1):31-45. Epub 2007 Dec 12.Substituted pyrazoles as hepatoselective HMG-CoA reductase inhibitors: discovery of (3R,5R)-7-[2-(4-fluoro-phenyl)-4-isopropyl-5-(4-methyl-benzylcarbamoyl)-2H-pyrazol-3-yl]-3,5-dihydroxyheptanoic acid (PF-3052334) as a candidate for the treatment of hypercholesterolemia. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

