Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0N8XL
|
||||
| Former ID |
DIB012523
|
||||
| Drug Name |
BMS-605339
|
||||
| Synonyms |
NS3 protease inhibitor (hepatitis C virus infection), BMS
|
||||
| Indication | HCV infection [ICD9: 070.4, 070.5, 070.70; ICD10:B17.1, B18.2] | Terminated | [1] | ||
| Company |
Bristol-Myers Squibb Co
|
||||
| Structure |
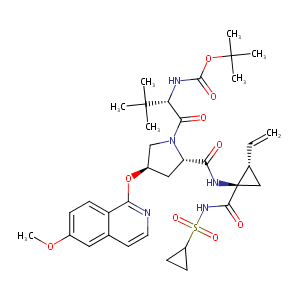
|
Download2D MOL |
|||
| Canonical SMILES |
[C@@H]1(C=C)C[C@@]1(C(=O)NS(=O)(=O)C1CC1)NC(=O)[C@@H]1C<br />[C@H](CN1C(=O)[C@H](C(C)(C)C)NC(=O)OC(C)(C)C)Oc1c2ccc(c<br />c2ccn1)OC
|
||||
| Target and Pathway | |||||
| Target(s) | Nonstructural protein NS3 | Target Info | Inhibitor | [2] | |
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800030836) | ||||
| REF 2 | Discovery and early clinical evaluation of BMS-605339, a potent and orally efficacious tripeptidic acylsulfonamide NS3 protease inhibitor for the treatment of hepatitis C virus infection. J Med Chem.2014 Mar 13;57(5):1708-29. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

