Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0OR2L
|
||||
| Former ID |
DNC002624
|
||||
| Drug Name |
Cholic Acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Peroxisomal disorders; Synthesis disorders | Approved | [541327] | ||
| Structure |
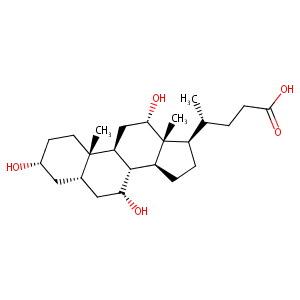
|
Download2D MOL |
|||
| Formula |
C24H40O5
|
||||
| InChI |
InChI=1S/C24H40O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18+,19-,20+,22+,23+,24-/m1/s1
|
||||
| InChIKey |
BHQCQFFYRZLCQQ-OELDTZBJSA-N
|
||||
| CAS Number |
CAS 81-25-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
3963, 72129, 584143, 607109, 822057, 841391, 3135999, 4266387, 7886587, 8145669, 9376190, 11110315, 14757556, 15277848, 17422064, 24423691, 24423929, 24892356, 24893153, 26717723, 26717726, 26717727, 26737120, 26737126, 26737128, 26750105, 26750407, 26750412, 26750415, 26750420, 26750424, 30424718, 46507063, 47193710, 47662491, 49700787, 50452100, 53787750, 53789207, 56313736, 56437567, 56437568, 57399957, 81067251, 81091088, 85279435, 85285851, 85285861, 85285865, 85285871
|
||||
| SuperDrug ATC ID |
A05AA03
|
||||
| Target and Pathway | |||||
| Target(s) | Phospholipase A2 | Target Info | Inhibitor | [551393] | |
| Ferrochelatase | Target Info | Inhibitor | [551393] | ||
| Liver carboxylesterase | Target Info | Inhibitor | [551393] | ||
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Ether lipid metabolism | |||||
| Arachidonic acid metabolism | |||||
| Linoleic acid metabolism | |||||
| alpha-Linolenic acid metabolism | |||||
| Metabolic pathways | |||||
| Ras signaling pathway | |||||
| Vascular smooth muscle contraction | |||||
| Pancreatic secretion | |||||
| Fat digestion and absorptionhsa00860:Porphyrin and chlorophyll metabolism | |||||
| Metabolic pathwayshsa00983:Drug metabolism - other enzymes | |||||
| PANTHER Pathway | Heme biosynthesis | ||||
| PathWhiz Pathway | Porphyrin Metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

