Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Q3QS
|
||||
| Former ID |
DIB013800
|
||||
| Drug Name |
4-aminosalicylate sodium (oral controlled release, ulcerative colitis), AGI Therapeutics
|
||||
| Synonyms |
AGI-022; Salicylate prodrug (oralcontrolled release, ulcerative colitis), AGI Therapeutics; 4-ASA-Na; 4-ASA-Na (oral, controlled release), AGI Therapeutics
|
||||
| Indication | Inflammatory bowel disease [ICD9: 555, 556; ICD10:K50, K51] | Phase 1 | [1] | ||
| Company |
Athpharma Ltd
|
||||
| Structure |
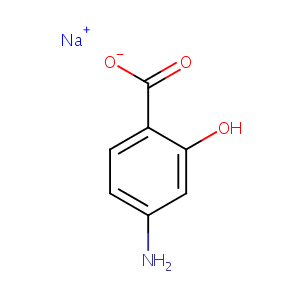
|
Download2D MOL |
|||
| Canonical SMILES |
c1(c(cc(cc1)N)O)C(=O)[O-].[Na+]
|
||||
| Target and Pathway | |||||
| Target(s) | Cyclooxygenase | Target Info | Modulator | [2] | |
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022712) | ||||
| REF 2 | 5-aminosalicylic acid mediates expression of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase to suppress colorectal tumorigenesis. Anticancer Res. 2012 Apr;32(4):1193-202. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

