Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0R3QY
|
||||
| Former ID |
DAP000557
|
||||
| Drug Name |
Vigabatrin
|
||||
| Synonyms |
GVG; Sabril; Sabrilex; Vigabatrina; Vigabatrine; Vigabatrinum; Aventis Brand of Vigabatrin; Gamma Vinyl GABA; Gamma Vinyl gamma Aminobutyric Acid; Hoechst Brand of Vigabatrin; Vigabatrin Aventis Brand; Vigabatrin Hoechst Brand; Vigabatrin Yamanouchi Brand; Vigabatrina [Spanish]; Vigabatrine [French]; Vigabatrinum [Latin]; Yamanouchi Brand of Vigabatrin; M071754; MDL 71754; RMI 71754; V 8261; V8261_SIGMA; CPP-109; Gamma-Vinyl GABA; MDL 71,754; MDL-71754; RMI-71754; RMI-71890; Sabril (TN); Sabrilex (TN); Gamma-Vinyl-GABA; Hexenoic acid, 4-amino; Vigabatrin [USAN:BAN:INN]; Vigabatrin [USAN:INN:BAN]; Gamma-Vinyl-gamma-Aminobutyric Acid; Gamma-Vinyl-gamma-aminobutyric acid; Vigabatrin (JAN/USAN/INN); Acid, gamma-Vinyl-gamma-Aminobutyric; (R,S)-4-Amino-5-hexenoic acid; (inverted question mark)-gamma-Vinyl GABA; 4-Amino-5-hexenoic acid; 4-Aminohexenoic acid; 4-aminohex-5-enoic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Epilepsy; Infantile spasms; Complex partial seizures [ICD9: 345, 345.4, 345.5, 345.9, 728.85, 780.3; ICD10:G40, G40.2, P90, R25.2, R56] | Approved | [1], [2] | ||
| Therapeutic Class |
Anticonvulsants
|
||||
| Company |
Ovation Pharma; Lundbeck
|
||||
| Structure |
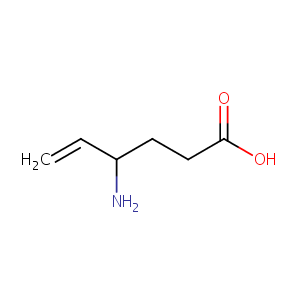
|
Download2D MOL |
|||
| Formula |
C6H11NO2
|
||||
| InChI |
InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)
|
||||
| InChIKey |
PJDFLNIOAUIZSL-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 60643-86-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9703, 634838, 5440157, 7847601, 7980880, 8153478, 10321780, 11341935, 11362118, 11363262, 11365824, 11368386, 11373068, 11376548, 11466529, 11467649, 11486078, 11487520, 11491640, 11494182, 12012774, 15194489, 17405831, 24278202, 26612272, 26747129, 26751903, 29224702, 46507052, 47217077, 47662561, 47960037, 47960038, 47960039, 48035425, 48334803, 48334804, 49698839, 49976606, 50104581, 50104582, 50104583, 53778383, 53788300, 56313009, 57322887, 79363997, 85231284, 90340732, 92125387
|
||||
| SuperDrug ATC ID |
N03AG04
|
||||
| SuperDrug CAS ID |
cas=060643869
|
||||
| Target and Pathway | |||||
| Target(s) | 4-aminobutyrate aminotransferase, mitochondrial | Target Info | Inhibitor | [2], [3], [4], [5] | |
| BioCyc Pathway | GABA shunt | ||||
| Valine degradation | |||||
| Beta-alanine degradation | |||||
| 4-aminobutyrate degradation | |||||
| KEGG Pathway | Alanine, aspartate and glutamate metabolism | ||||
| Valine, leucine and isoleucine degradation | |||||
| beta-Alanine metabolism | |||||
| Propanoate metabolism | |||||
| Butanoate metabolism | |||||
| Metabolic pathways | |||||
| GABAergic synapse | |||||
| PANTHER Pathway | Aminobutyrate degradation | ||||
| Pyrimidine Metabolism | |||||
| Gamma-aminobutyric acid synthesis | |||||
| PathWhiz Pathway | Aspartate Metabolism | ||||
| Glutamate Metabolism | |||||
| Beta-Alanine Metabolism | |||||
| Valine, Leucine and Isoleucine Degradation | |||||
| Propanoate Metabolism | |||||
| WikiPathways | GABA synthesis, release, reuptake and degradation | ||||
| Alanine and aspartate metabolism | |||||
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4821). | ||||
| REF 2 | Hughes B: 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89-92. | ||||
| REF 3 | Gamma-vinyl GABA, an irreversible inhibitor of GABA transaminase, alters the acquisition and expression of cocaine-induced sensitization in male rats. Synapse. 2002 Dec 15;46(4):240-50. | ||||
| REF 4 | Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006 Feb;6(1):7-17. | ||||
| REF 5 | Vigabatrin for refractory complex partial seizures: multicenter single-blind study with long-term follow-up. Neurology. 1987 Feb;37(2):184-9. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

