Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0SU1G
|
||||
| Former ID |
DIB000387
|
||||
| Drug Name |
Zomepirac
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89] | Withdrawn from market | [551871] | ||
| Structure |
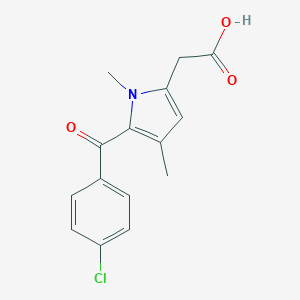
|
Download2D MOL |
|||
| Formula |
C15H17ClNNaO5
|
||||
| Canonical SMILES |
Cc1cc(CC(=O)O)n(C)c1C(=O)c2ccc(Cl)cc2
|
||||
| InChI |
1S/C15H14ClNO3/c1-9-7-12(8-13(18)19)17(2)14(9)15(20)10-3-5-11(16)6-4-10/h3-7H,8H2,1-2H3,(H,18,19)
|
||||
| InChIKey |
ZXVNMYWKKDOREA-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 33369-31-2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cyclooxygenase | Target Info | Modulator | [533421], [551871] | |
| References | |||||
| Ref 533421 | Effect of preischemia cyclooxygenase inhibition by zomepirac sodium on reflow, cerebral autoregulation, and EEG recovery in the cat after global ischemia. J Cereb Blood Flow Metab. 1986 Dec;6(6):691-702. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

