Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T2MN
|
||||
| Former ID |
DIB000771
|
||||
| Drug Name |
Z-300
|
||||
| Synonyms |
2-(2-Hydroxyethylsulfanyl)-N-[3-[3-(piperidin-1-ylmethyl)phenoxy]propyl]acetamide 2-(4-hydroxybenzoyl)benzoate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Gastric ulcer [ICD9: 531; ICD10:K25] | Discontinued in Phase 1 | [1] | ||
| Structure |
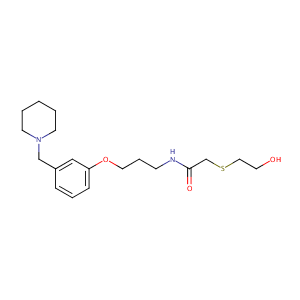
|
Download2D MOL |
|||
| Formula |
C19H30N2O3S
|
||||
| Canonical SMILES |
C1CCN(CC1)CC2=CC(=CC=C2)OCCCNC(=O)CSCCO
|
||||
| InChI |
1S/C19H30N2O3S/c22-11-13-25-16-19(23)20-8-5-12-24-18-7-4-6-17(14-18)15-21-9-2-1-3-10-21/h4,6-7,14,22H,1-3,5,8-13,15-16H2,(H,20,23)
|
||||
| InChIKey |
ZRVDLLKTYZXNAD-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 127966-78-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Histamine H2 receptor | Target Info | Antagonist | [2], [3] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Gastric acid secretion | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| Histamine H2 receptor mediated signaling pathway | |||||
| PathWhiz Pathway | Intracellular Signalling Through Histamine H2 Receptor and Histamine | ||||
| Gastric Acid Production | |||||
| Reactome | Histamine receptors | ||||
| G alpha (s) signalling events | |||||
| WikiPathways | Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| Secretion of Hydrochloric Acid in Parietal Cells | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001448) | ||||
| REF 2 | Effects of a new histamine H2-receptor antagonist, Z-300, on gastric secretion and gastro-duodenal lesions in rats: comparison with roxatidine. Jpn J Pharmacol. 1992 Jul;59(3):275-89. | ||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

