Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0U8AT
|
||||
| Former ID |
DAP000962
|
||||
| Drug Name |
Echothiophate Iodide
|
||||
| Synonyms |
Echodide; Phospholine; Diethoxyphosphinylthiocholine iodide; ECOTHIOPATE IODIDE; Ecostigmini jodidum; Ecothiopati iodidum; Ecothiophate iodide; Ioduro de ecotiopato; Phospholine iodide; Diethoxyphosphoryl-thiocholine iodide; Echothiophate iodide (USP); Ecothiopati iodidum [INN-Latin]; Iodide, Echothiophate; Iodide, Ecothiopate; Iodide, Ecothiophate; Iodide, Phospholine; Iodure d'ecothiopate; Ioduro de ecotiopato [INN-Spanish]; Phospholine (the pharmaceutical); Phospholine Iodide (TN); Phospholine iodide (TN); Ecothiopate iodide (JP15/INN); Iodure d'ecothiopate [INN-French]; N-(2-(Diethoxyphosphinylthio)ethyl)trimethylammonium iodide; O,O-Diethyl S-2-trimethylammonium ethylphosphonothiolate iodide; S-Ester of (2-mercaptoethyl)trimethylammonium iodide with O,O-diethyl phosphorothioate; S-beta-dimethylaminoethyl-O,O-diethylthionophosphate methiodide; S-(2-Dimethylaminoethyl)-O.O-diethylphosphorothioate methiodide; S-(2-dimethylaminoethyl)-O,O-diethylphosphorothioate methiodide; Ammonium, (2-(O,O-diethylphosphorothio)ethyl)trimethyl-, iodide; Ammonium, (2-mercaptoethyl)trimethyl-, iodide, S-ester with O,O-diethylphosphorothioate; S-(2-(N,N,N-Trimethylammonio)ethyl) O,O-diethylphosphorothiolate iodide; Ethanaminium, 2-((diethoxyphosphinyl)thio)-N,N,N-trimethyl-, iodide; (2-Mercaptoethyl)trimethylammonium iodide S-ester with O,O-diethyl phosphorothioate; (2-mercaptoethyl)trimethylammonium iodidie O,O-diethyl phosphorothioate; 2-((Diethoxyphosphinyl)thio)-N,N,N,-trimethylethanaminium iodide; 2-Diaethoxyphosphinyl-thioaethyl-trimethyl-ammonium-jodid; 2-Diaethoxyphosphinyl-thioaethyl-trimethyl-ammonium-jodid [German]; 2-Diethoxy-phosphinylthioethyl-trimethylammonium iodide; 2-[(diethoxyphosphinyl)thio]-N,N,N-trimethylethanaminium iodide; 2-[(diethoxyphosphoryl)sulfanyl]-N,N,N-trimethylethanaminium iodide; 2-diethoxyphosphorylsulfanylethyl(trimethyl)azanium iodide; 2-{[bis(ethyloxy)phosphoryl]thio}-N,N,N-trimethylethanaminium iodide; 217 MI; 217-mi
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Chronic glaucoma [ICD9: 365; ICD10:H40-H42] | Approved | [538454] | ||
| Therapeutic Class |
Parasympathomimetics
|
||||
| Company |
Wyeth Pharmaceuticals
|
||||
| Structure |
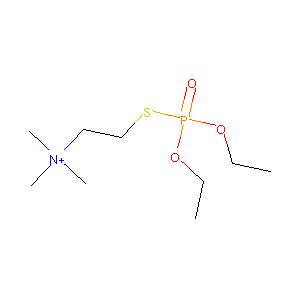
|
Download2D MOL |
|||
| Formula |
C9H23INO3PS
|
||||
| Canonical SMILES |
CCOP(=O)(OCC)SCC[N+](C)(C)C.[I-]
|
||||
| InChI |
1S/C9H23NO3PS.HI/c1-6-12-14(11,13-7-2)15-9-8-10(3,4)5;/h6-9H2,1-5H3;1H/q+1;/p-1
|
||||
| InChIKey |
OVXQHPWHMXOFRD-UHFFFAOYSA-M
|
||||
| CAS Number |
CAS 6736-03-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
S01EB03
|
||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Modulator | [556264] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

