Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V1UW
|
||||
| Former ID |
DAP000774
|
||||
| Drug Name |
Nitisinone
|
||||
| Synonyms |
Nitisinona; Nitisinonum; Nitisone; Orfadin; Nitisinone [INN]; SC 0735; FE-0200; Nitisinone (NTBC); Nitisinone [USAN:INN]; Orfadin (TN); Nitisinone (JAN/USAN/INN); 2-(2-Nitro-4-trifluoromethylbenzoyl)cyclohexane-1,3-dione; 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione; 2-(Alpha,alpha,alpha-trifluoro-2-nitro-p-tuluoyl)-1,3-cyclohexanedione; 2-[2-nitro-4-(trifluoromethyl)benzoyl]cyclohexane-1,3-dione; 2-{[2-nitro-4-(trifluoromethyl)phenyl]carbonyl}cyclohexane-1,3-dione
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
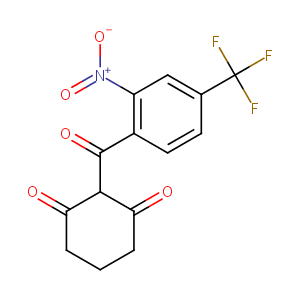
|
Download2D MOL |
|||
| Formula |
C14H10F3NO5
|
||||
| InChI |
InChI=1S/C14H10F3NO5/c15-14(16,17)7-4-5-8(9(6-7)18(22)23)13(21)12-10(19)2-1-3-11(12)20/h4-6,12H,1-3H2
|
||||
| InChIKey |
OUBCNLGXQFSTLU-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 104206-65-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10236750, 14753104, 29296848, 46507380, 47206901, 49957875, 50139362, 56311852, 57339133, 76271421, 93167147, 102979918, 103425313, 103987675, 104398189, 123051096, 124758249, 124896666, 125432673, 126666671, 128861885, 131293388, 134223796, 134338059, 135072534, 136340345, 137003783, 140881929, 143500522, 152030782, 152134009, 152227462, 152248599, 152344367, 160821612, 160963695, 162011463, 162165008, 162203592, 163091628, 163372627, 163775323, 164045265, 164175197, 164216726, 164763350, 165702339, 174007248, 174525942, 175268251
|
||||
| ChEBI ID |
ChEBI:50378
|
||||
| SuperDrug ATC ID |
A16AX04
|
||||
| SuperDrug CAS ID |
cas=104206657
|
||||
| Target and Pathway | |||||
| Target(s) | 4-hydroxyphenylpyruvate dioxygenase | Target Info | Inhibitor | [535880], [536692] | |
| References | |||||
| Ref 538562 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021232. | ||||
| Ref 541915 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6834). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

