Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0W8SJ
|
||||
| Former ID |
DAP000475
|
||||
| Drug Name |
Minaprine
|
||||
| Synonyms |
Brantur; Cantor; Minaprina; Minaprinum; Minaprine dihydrochloride; AGR 1240; CB 30038; Cantor (TN); Minaprina [INN-Spanish]; Minaprinum [INN-Latin]; Minaprine (USAN/INN); Minaprine [USAN:BAN:INN]; N-(4-Methyl-6-phenyl-3-pyridazinyl)-4-morpholineethanamine; 3-(morpholinoethyl)amino-4-methyl-6-phenylpyridazine; 4-(2-((4-Methyl-6-phenyl-3-pyridazinyl)amino)ethyl)morpholine; 4-Methyl-3-(2-morpholinoethylamino)-6-phenylpyridazin; 4-methyl-N-(2-morpholin-4-ylethyl)-6-phenylpyridazin-3-amine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antidepressants
|
||||
| Structure |
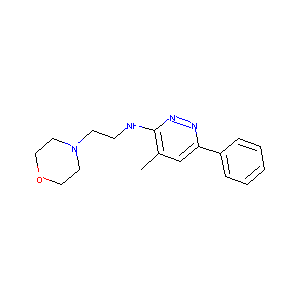
|
Download2D MOL |
|||
| Formula |
C17H22N4O
|
||||
| Canonical SMILES |
CC1=CC(=NN=C1NCCN2CCOCC2)C3=CC=CC=C3
|
||||
| InChI |
1S/C17H22N4O/c1-14-13-16(15-5-3-2-4-6-15)19-20-17(14)18-7-8-21-9-11-22-12-10-21/h2-6,13H,7-12H2,1H3,(H,18,20)
|
||||
| InChIKey |
LDMWSLGGVTVJPG-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 25905-77-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7433695, 8152626, 11112706, 11335238, 11360477, 11364228, 11366790, 11369352, 11372508, 11373855, 11377514, 11461449, 11466094, 11467214, 11485190, 11485908, 11489223, 11491260, 11492233, 11495148, 14751646, 24896803, 29223304, 46505380, 47206766, 47216618, 47290970, 47440078, 47588828, 47662107, 47959567, 47959568, 49698916, 49993893, 50100510, 56394929, 57322189, 57654369, 61212480, 77581144, 85209879, 85787830, 103140727, 103194634, 103967506, 104024299, 104305716, 117523812, 124599829, 124882544
|
||||
| ChEBI ID |
ChEBI:51038
|
||||
| SuperDrug ATC ID |
N06AX07
|
||||
| SuperDrug CAS ID |
cas=025905775
|
||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 2B receptor | Target Info | Antagonist | [535814], [536549] | |
| PANTHER Pathway | 5HT2 type receptor mediated signaling pathway | ||||
| References | |||||
| Ref 535814 | Protective effect of minaprine against the abnormal changes of 2-deoxyglucose uptake by rat hippocampal slices induced by hypoxia/hypoglycemia. Jpn J Pharmacol. 1992 Sep;60(1):33-8. | ||||
| Ref 536549 | Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini Rev Med Chem. 2007 Nov;7(11):1108-19. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

