Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0BA7Q
|
||||
| Former ID |
DNC011603
|
||||
| Drug Name |
1-Methyl-8-phenyl-3,7-dihydro-purine-2,6-dione
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [533339] | ||
| Structure |
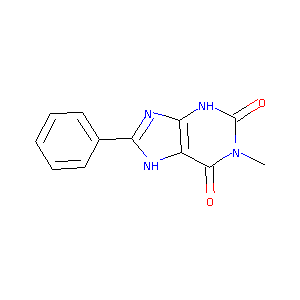
|
Download2D MOL |
|||
| Formula |
C12H10N4O2
|
||||
| Canonical SMILES |
CN1C(=O)C2=C(NC1=O)N=C(N2)C3=CC=CC=C3
|
||||
| InChI |
1S/C12H10N4O2/c1-16-11(17)8-10(15-12(16)18)14-9(13-8)7-5-3-2-4-6-7/h2-6H,1H3,(H,13,14)(H,15,18)
|
||||
| InChIKey |
UOOOWRVLSKCKGJ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Adenosine A2a receptor | Target Info | Inhibitor | [526958] | |
| Adenosine A1 receptor | Target Info | Inhibitor | [533339] | ||
| NetPath Pathway | TCR Signaling Pathway | ||||
| RANKL Signaling Pathway | |||||
| Pathway Interaction Database | HIF-2-alpha transcription factor network | ||||
| References | |||||
| Ref 526958 | J Med Chem. 2004 Feb 12;47(4):1031-43.Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugswith peroral bioavailability. | ||||
| Ref 533339 | J Med Chem. 1989 Jun;32(6):1231-7.Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di-, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

