Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03DJL
|
||||
| Former ID |
DCL000846
|
||||
| Drug Name |
Indacaterol
|
||||
| Synonyms |
QAB-149; Indacaterol (USAN/INN); 5-(2-(5,6-Diethylindan-2-ylamino)-1-hydroxyethyl)-8-hydroxy-1H-quinolin-2-one; 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Novartis
|
||||
| Structure |
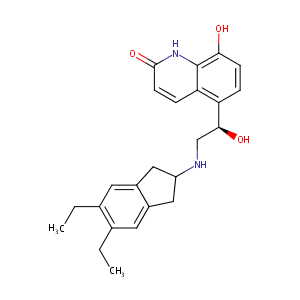
|
Download2D MOL |
|||
| Formula |
C24H28N2O3
|
||||
| InChI |
InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1
|
||||
| InChIKey |
QZZUEBNBZAPZLX-QFIPXVFZSA-N
|
||||
| CAS Number |
CAS 312753-06-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
14805326, 15126362, 17194957, 43529924, 50944939, 96025998, 103751149, 104152520, 114788160, 126665799, 134339311, 135252041, 136946521, 137248550, 137261964, 140383548, 144116077, 152258566, 152343993, 160645865, 160647401, 162011653, 162197410, 164227489, 164777215, 172919100, 174528841, 175266645, 175426936, 187051758, 189561508, 198991946, 223601296, 223667503, 223701040, 224342928, 226432041, 241382349, 246478537, 247088325, 251916861, 251918100, 252063646, 252110200, 252436254
|
||||
| SuperDrug ATC ID |
R03AC18
|
||||
| Target and Pathway | |||||
| Target(s) | Beta-2 adrenergic receptor | Target Info | Agonist | [536223], [537130] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | ||||
| Arf6 signaling events | |||||
| References | |||||
| Ref 531783 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | ||||
| Ref 542479 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7455). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

