Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05KBP
|
||||
| Former ID |
DIB001403
|
||||
| Drug Name |
INO-8875
|
||||
| Synonyms |
PJ-875; Adenosine A1 agonist (atrial fibrillation), Inotek; Adenosine A1 agonist (glaucoma), Inotek
|
||||
| Indication | Glaucoma [ICD9: 365; ICD10:H40-H42] | Phase 1/2 | [523044] | ||
| Company |
Inotek pharmaceuticals
|
||||
| Structure |
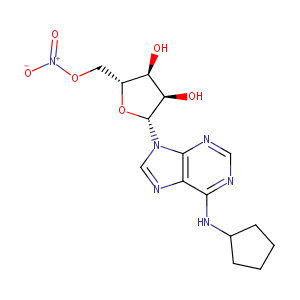
|
Download2D MOL |
|||
| Canonical SMILES |
C1CCCC1Nc1ncnc2n([C@H]3[C@H](O)[C@H](O)[C@@H](CO[N+](=O<br />)[O-])O3)cnc12
|
||||
| Target and Pathway | |||||
| Target(s) | Adenosine A1 receptor | Target Info | Agonist | [532076] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| RANKL Signaling Pathway | |||||
| References | |||||
| Ref 523044 | ClinicalTrials.gov (NCT01123785) A Dose-Escalation Study Designed to Evaluate the Tolerability, Safety, Pharmacokinetics (PK), and Efficacy of Chronic Topical Ocular Application of INO-8875 in AdultsWith Ocular Hypertension or Primary Open-Angle Glaucoma. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

