Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01SYU
|
||||
| Former ID |
DNCL001781
|
||||
| Drug Name |
Turofexorate isopropyl
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Hyperlipidaemia [ICD9: 272.0-272.4; ICD10:E78] | Phase 1 | [548483] | ||
| Company |
Pfizer New York, NY
|
||||
| Structure |
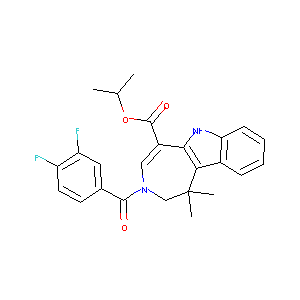
|
Download2D MOL |
|||
| Formula |
C25H24F2N2O3
|
||||
| Canonical SMILES |
CC(C)OC(=O)C1=CN(CC(C2=C1NC3=CC=CC=C32)(C)C)C(=O)C4=CC(<br />=C(C=C4)F)F
|
||||
| InChI |
1S/C25H24F2N2O3/c1-14(2)32-24(31)17-12-29(23(30)15-9-10-18(26)19(27)11-15)13-25(3,4)21-16-7-5-6-8-20(16)28-22(17)21/h5-12,14,28H,13H2,1-4H3
|
||||
| InChIKey |
INASOKQDNHHMRE-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
15007710, 22454421, 44143203, 58097730, 78432654, 85852616, 92729644, 96024964, 103591352, 123055418, 126671459, 135261047, 136349558, 136367689, 137275949, 137348407, 152258858, 160647707, 162038105, 162156117, 162202780, 163395032, 163843769, 165246682, 184825294, 189622847, 198989171, 202555789, 223386908, 223612979, 223676985, 223705224, 223850150, 226620161, 242060100, 248959552, 249866596, 250212671, 251971163, 252074945, 252160417, 252216607, 252438376
|
||||
| Target and Pathway | |||||
| Target(s) | Bile acid receptor | Target Info | Modulator | [529912] | |
| KEGG Pathway | Bile secretion | ||||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

