Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T3DO
|
||||
| Former ID |
DIB003449
|
||||
| Drug Name |
IRX-5183
|
||||
| Synonyms |
AGN-195183; NRX-195183; NRX-5183; VTP-195183; VTP-5183; IRX-5183 (oral, autoimmune disease), Io Therapeutics; RARa (oral, cancer/autoimmune disease), Io Therapeutics; RARa agonist (topical, skin/eye diseases), Io Therapeutics; Retinoic acid receptor alpha agonist (oral, cancer/autoimmune disease), Io Therapeutics; Retinoic acid receptor alpha agonist (topical, skin/eye diseases), Io Therapeutics; IRX-5183 (oral, cancer/autoimmune disease), Io Therapeutics; IRX-5183 (topical, skin/eye diseases), Io Therapeutics
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Discontinued in Phase 2 | [547464] | ||
| Company |
Vitae Pharmaceuticals
|
||||
| Structure |
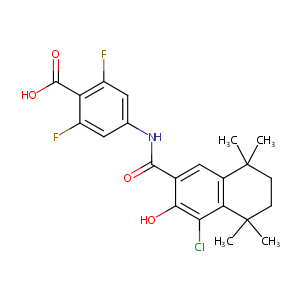
|
Download2D MOL |
|||
| Canonical SMILES |
c1(C(=O)Nc2cc(c(c(c2)F)C(=O)O)F)c(c(c2c(c1)C(CCC2(C)C)(<br />C)C)Cl)O
|
||||
| CAS Number |
CAS 367273-07-2
|
||||
| Target and Pathway | |||||
| Target(s) | Retinoic acid receptor alpha | Target Info | Agonist | [526923], [529087] | |
| Pathway Interaction Database | Retinoic acid receptors-mediated signaling | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

